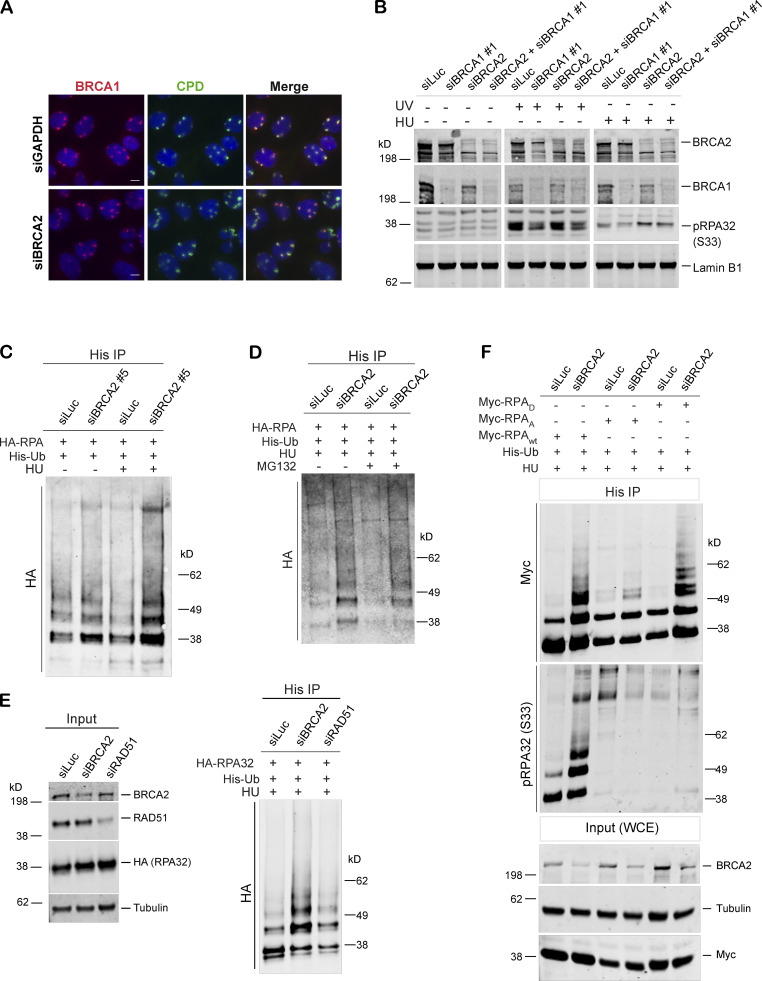
Figure S2.
BRCA1 may function upstream of BRCA2 in the stalled fork repair pathway, and BRCA2 depletion results in hyperubiquitination of RPA after stalled fork–inducing DNA damage. (A) BRCA1 recruitment was not affected in cells depleted of BRCA2. IF analysis of BRCA1 recruitment in U2OS control cells and BRCA2-depleted cells after UV irradiation as indicated above. Cells were costained with BRCA1 and CPD. Scale bars indicate 10 µm. (B) Western blot analysis of RPA32 accumulation in U2OS cells transfected with indicated siRNAs. (C) Immunoprecipitation analysis of RPA32 ubiquitination in HEK293T cells transfected with siLuc or a different BRCA2-specific siRNA (siBRCA2#5). (D) Immunoprecipitation analysis of RPA ubiquitination in HEK293T control and BRCA2-deficient cells in the absence or presence of MG132. HEK293T cells with indicated siRNAs were transfected with His-tagged ubiquitin and HA-tagged RPA32. Cells were treated with 5mM HU for 3 h. Before harvesting the cells, 10 µM MG132 was added for 1 h. (E) Immunoprecipitation analysis of RPA ubiquitination in HEK293T cells transfected with control (siLuc) or BRCA2 orRAD51 siRNAs. Experimental conditions used are as described before. (F) Immunoprecipitation analysis to study the relationship between RPA phosphorylation and ubiquitination by expressing various RPA mutants. In RPAD mutant, both of the cyclin-cdk2 phosphorylation sites and six of the stress-dependent phosphorylation sites (S8, S11, S12, S13, T21, and S33) were replaced by aspartate. In RPAA mutant, these same sites were converted to alanine to prevent phosphorylation. HEK293T cells were transfected with indicated siRNAs, followed by transfection with His-tagged ubiquitin and Myc-tagged WT or RPAA, RPAD mutant RPA. Cells were processed for His immunoprecipitation as described above. Blot was probed with anti-Myc and anti-pRPA32 (S33) antibodies. Cells were collected after 4 h of HU treatment (5 mM). A different BRCA1-specific siRNA (siBRCA1#1) was used in B. Related to Figs. 2 and 4.