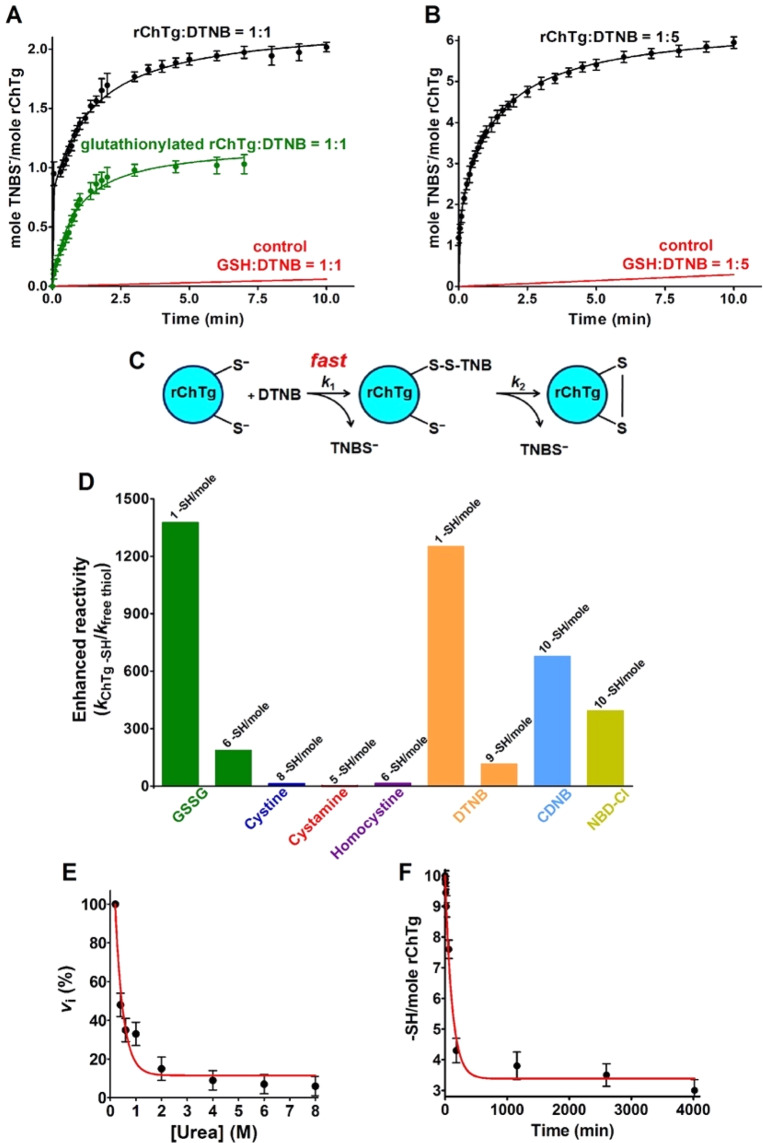
Figure 2.
Reactivity of rChTg cysteines. (A) TNBS- release after reaction of rChTg (0.58 µM, 5.8 µM protein -SH) with substoichiometric DTNB (0.58 µM) in 50 mM acetate buffer at pH 5.0, 0.2 M urea 25 °C (black line). The same rection as above but after 20 min incubation of 0.58 µM of rChTg with 1 mM GSSG (green line). Reaction of GSH (5.8 µM) with DTNB (0.58 µM) in the same conditions (red line). (B) TNBS- release after reaction of rChTg (0.58 µM, 5.8 µM protein -SH) with substoichiometric DTNB (2.9 µM) in 50 mM acetate buffer at pH 5.0, 0.2 M urea 25 °C. Reaction of GSH (5.8 µM) with DTNB (2.9 µM) in the same conditions (red line). (C) Schematic representation of the reaction of rChTg with stoichiometric DTNB. (D) “Enhanced reactivity” of rChTg toward disulfides and thiol reagents i.e. second order kinetic constants of rChTg (kChTg -SH) normalized to the constant calculated for an unperturbed protein cysteine for GSSG or normalized to the constants for free GSH for all other reagents (kfree thiol) (see Table 1). The number of protein cysteines per mole with a given reactivity is indicated on the top of each column. (E) Reactivity of cysteines in rChTg (0.6 µM) toward DTNB (47.5 μM) at variable urea concentrations (pH 5.0) (25 °C) (circles, red line). Rate of reaction of 10 µM free cysteine (or GSH) with 50 µM DTNB was not inhibited by 8 M urea (not shown). The error bars represent the S.D. from three independent experiments. (F) Disappearence of rChTg cysteines (5 µM, 50 µM protein -SH) during the reaction with 1 mM GSSG at pH 5.0, 0.2 M urea (25 °C). The error bars represent the S.D. from three independent experiments.