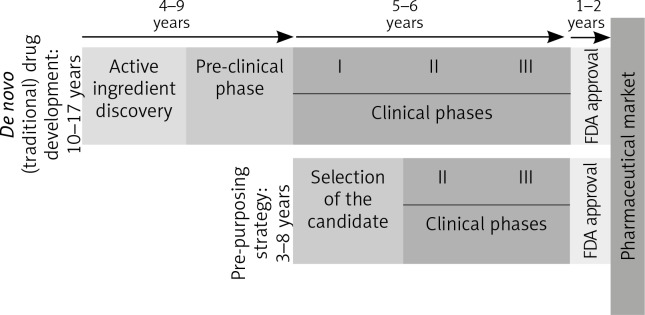
Fig. 2.
De novo and re-purposing drug development phases. Unlike the drugs that follow the conventional pathway to the pharmaceutical market, re-purposing candidates shorten the time needed to market by omitting some initial steps, which go directly into the clinical study phases (FDA – Food and Drug Administration. I, II, III – stages in the clinical development [clinical phases])