Fig. 3.8.
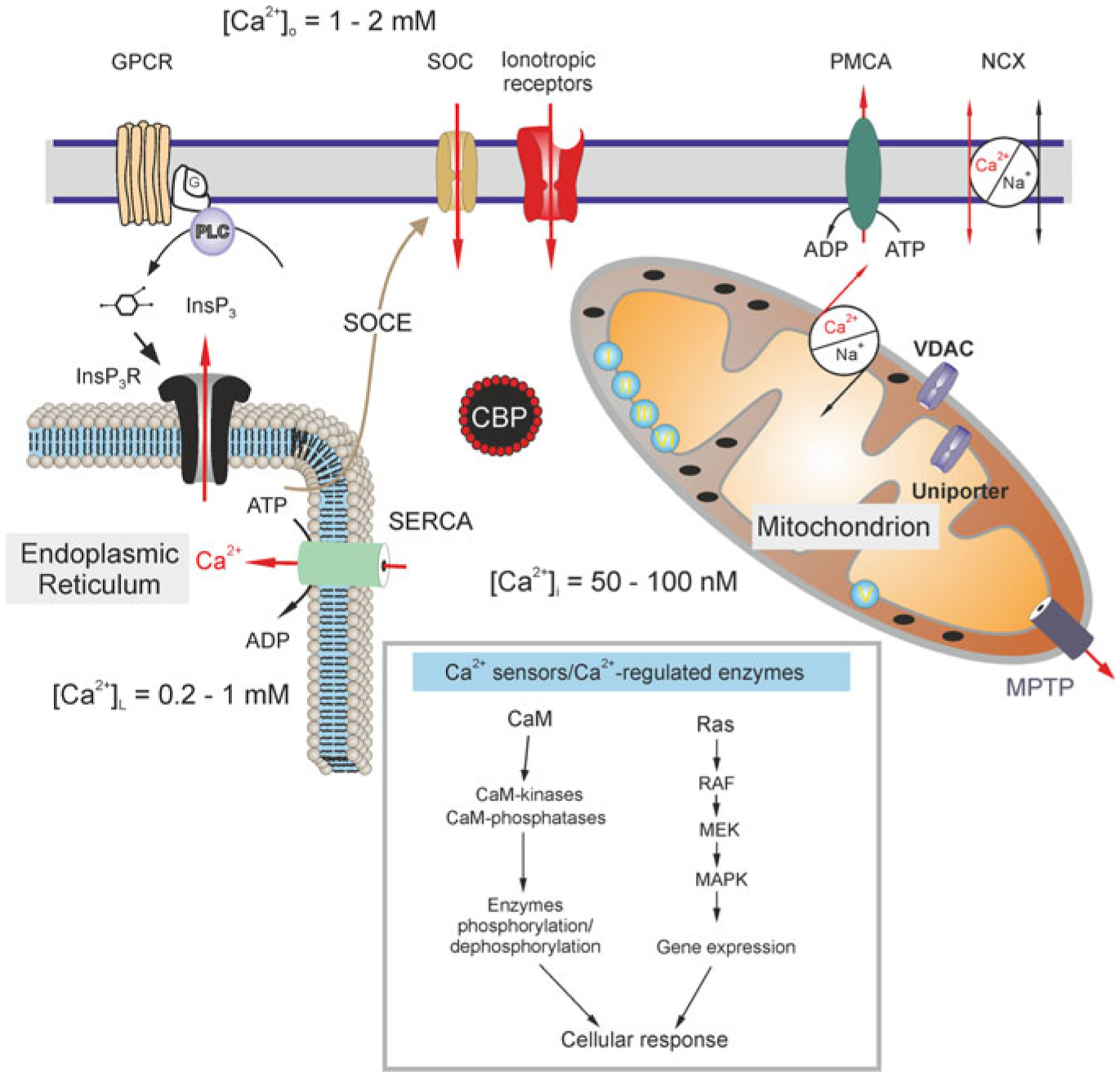
Calcium distribution and calcium signalling cascades in intracellular compartments. Stimuli-induced increases in [Ca2+]i could be caused by the entry of Ca2+ from the extracellular space through ionotropic receptors or store-operated channels (SOC). Plasmalemmal Ca2+ pumps/ATPases (PMCA) can extrude cytosolic Ca2+, while the plasmalemmal sodium-calcium exchanger (NCX) can operate in both directions depending on intercellular Na+ concentration and membrane potential. An additional source of Ca2+ is available from the ER internal store that possesses inositol 1,4,5 trisphosphate (InsP3) receptors, which can be activated by the activity of metabotropic G-protein coupled receptors (GPCRs) and phospholipase C (PLC). The ER store is (re)filled by the activity of the store-specific Ca2+-ATPase (SERCA). Cytosolic Ca2+ levels can be affected by a variety of cytosolic Ca2+-binding proteins (CBPs) and by the action of mitochondria. A negative membrane potential exists across the inner mitochondrial membrane. Mitochondrial Ca2+ uptake occurs through voltage-dependent anion channels (VDACs) present in the outer membrane and by the uniporter in the inner membrane as the electrochemical gradient drives Ca2+ into the matrix, while free Ca2+ exits the mitochondrial matrix through the mitochondrial Na+/Ca2+ exchanger and transient opening of the mitochondrial permeability transition pore (MPTP). Concentrations of free Ca2+ in different compartments are indicated on the scheme. Inset shows various Ca2+ effector molecules, sensors and enzymes. CaM, calmodulin; RAF, Rapidly Accelerated Fibrosarcoma, MAPK, mitogen-activated protein kinase (MAPK), MEK, MAPK kinase. Modified from [413]
