FIGURE 6.
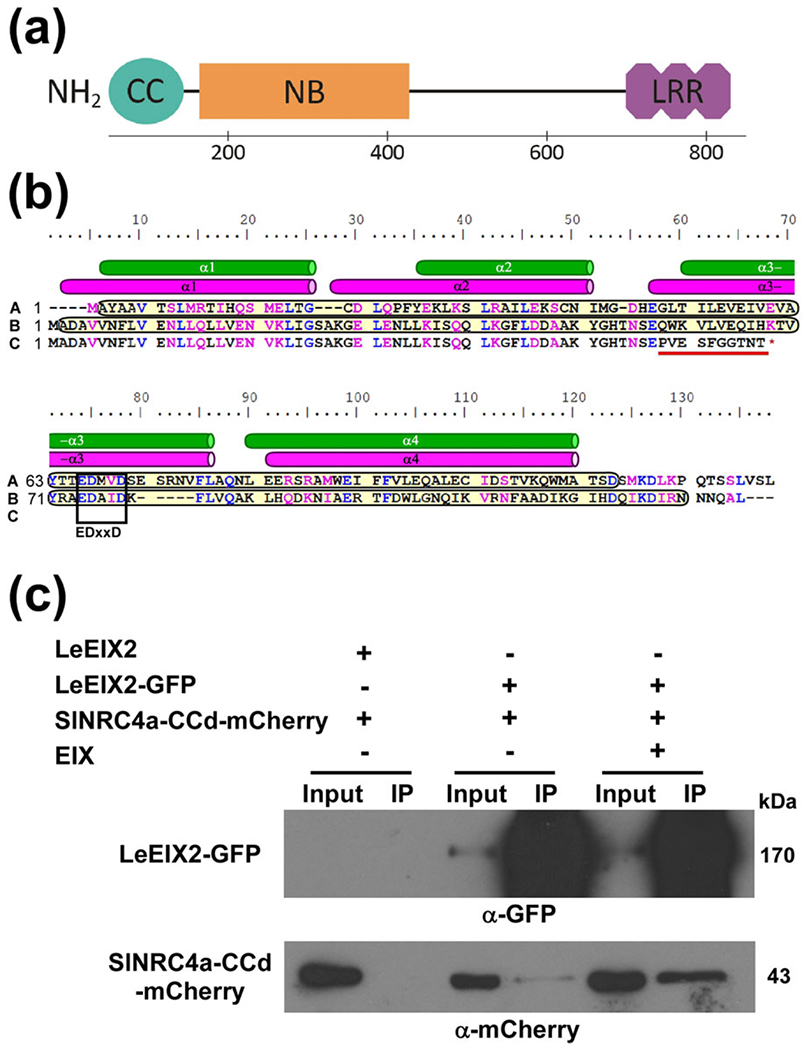
SlNRC4a’s coiled-coil domain associates with LeEIX2 in planta. (a) Schematic representation of SlNRC4a’s domain architecture. The Sol Genomics Network (Fernandez-Pozo et al., 2015) was used to plot the SlNRC4a protein sequence. Domains were identified using NCBI-conserved domains: coiled-coil 1–125 aa, nucleotide binding 159–437 aa, and leucine-rich repeat 696–838 aa (Marchler-Bauer et al., 2017). (b) SlNRC4a’s coiled-coil domain secondary structure. Sequence alignment of StRx 1–130 aa (A), SlNRC4a 1–130 aa (B), and slnrc4a-2 1–67 aa (C). Similar and identical aa are marked in magenta and blue, respectively. NCBI prediction of coiled-coil domains is marked in yellow (Marchler-Bauer et al., 2017). StRx-resolved and SlNRC4a-predicted α-helices shown as green and magenta cylinders, respectively (Hao et al., 2013). Conserved EDxxD motif is boxed. Mutated aa are underlined in red. (c) Co-IP of SlNRC4a’s coiled-coil domain with LeEIX2 in Nicotiana benthamiana. Plants were transiently cotransformed with LeEIX2-GFP or LeEIX2 and SlNRC4a-CCd-mCherry. Leaves were harvested and treated with either EIX or water (mock) at the petiole for 15 min. Triton X-100 soluble membrane protein fractions were immunopurified using GFP affinity beads. Input and immunopurified (IP) proteins were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis and immunoblot with anti-GFP and anti-mCherry
