Fig. 1.
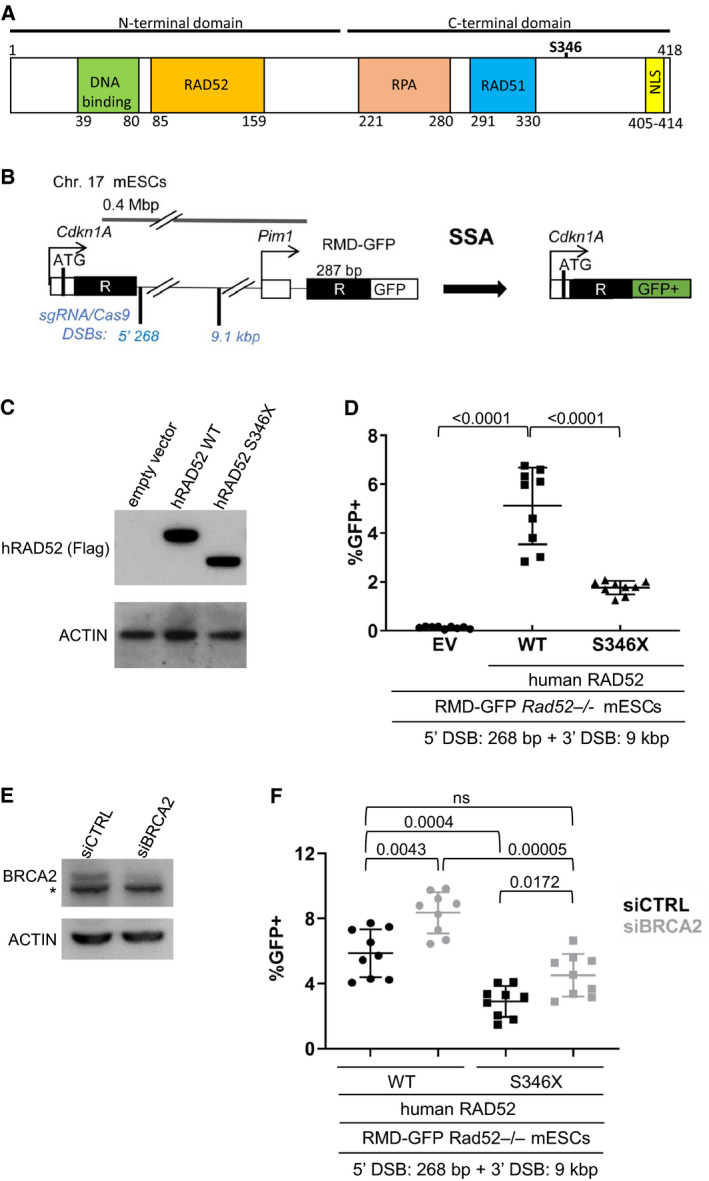
RAD52 S346X is dysfunctional in promoting SSA. (A) The domain map of human RAD52. The N‐terminal domain (1–212) contains DNA binding and self‐association regions. The C‐terminal domain (213–418) contains RPA and RAD51 interacting regions and a nuclear localization signal (NLS). (B) Diagram of the RMD‐GFP SSA reporter. Two tandem repeat (R) sequences are separated by 0.4 Mb and positioned such that SSA generates a Cdkn1a‐GFP fusion gene. SSA is induced by two DSBs between the repeats, with one DSB downstream from the 5′ repeat (5’268) and a second DSB 9.1 kbp upstream of the 3′ repeat. Shown are the single‐guide RNA (sgRNA)/Cas9 targeting sites for each DSB. Not to scale. (C) Flag immunoblot showing expression Flag‐hRAD52 WT and Flag‐hRAD52 S346X in RMD‐GFP Rad52−/− mESCs. (D) hRAD52 S346X is able to promote SSA but with a > 2‐fold decrease as compared to hRAD52 WT. Rad52−/− mESCs with RMD‐GFP were transfected with the 268 bp and 9 kbp sgRNA/Cas9 expression vectors along with a control EV, Flag‐hRAD52, or Flag‐hRAD52 S346X complementation vectors. Shown is the percentage of GFP+ cells from this experiment, normalized to transfection efficiency. n = 9. Lines represent the mean with SD. The numerical comparisons represent the P‐value determined using multiple t‐test with Holm–Sidak correction. (E) BRCA2 immunoblot showing depletion of BRCA2 in RMD‐GFP Rad52−/− mESCs, transfected with a pool of four BRCA2 siRNAs (siBRCA2). (∗) Nonspecific band. (F) Depletion of BRCA2 causes an increase in the ability of hRAD52 WT to promote SSA. RMD‐GFP Rad52−/− mESCs were transfected with the 268 bp and 9 kbp sgRNA/Cas9 expression vectors, either Flag‐hRAD52 WT or Flag‐hRAD52 S346X complementation vectors, along with a nontargeting siRNA (siCTRL) or siBRCA2. Shown is the percentage of GFP+ cells from this experiment, normalized to transfection efficiency. n = 9. Lines represent the mean with SD. The numbers shown above each comparison represent the P‐value determined using multiple t‐test with Holm–Sidak correction. (ns) Not significant.
