Background.
Pneumococcal conjugate vaccination as well as pneumococcal polysaccharide vaccination are recommended for lung transplant candidates and recipients, but the combination of these vaccines has not been extensively studied in these specific populations.
Methods.
Lung transplant candidates and recipients were vaccinated with a 13-valent pneumococcal conjugate vaccine, followed 8 weeks later by a pneumococcal polysaccharide vaccine. Pneumococcal antibody levels against 13 pneumococcal serotypes were measured and followed up after 1 year in the transplant recipients. These values were compared with a historical control group vaccinated with the polysaccharide vaccine alone.
Results.
Twenty-five lung transplant candidates and 23 lung transplant recipients were included. For the majority of serotypes, there was no significant increase in antibody levels after additional vaccination with the polysaccharide vaccine in both patient groups. When compared with the historical control group, the antibody response in lung transplant recipients 1 year after vaccination did not seem to have improved by vaccination with both vaccines instead of the polysaccharide vaccine alone.
Conclusions.
Serologic vaccination responses in lung transplant candidates and recipients were not improved by giving a 23-valent pneumococcal polysaccharide vaccine after a 13-valent pneumococcal conjugate vaccine. The benefit of this vaccination schedule in lung transplant recipients seems to differ from other immunocompromised populations. The optimal vaccination schedule for lung transplant candidates and recipients remains to be determined.
INTRODUCTION
Current recommendations for patients awaiting lung transplantation and lung transplant recipients state that they should receive pneumococcal conjugate vaccine as well as pneumococcal polysaccharide vaccine.1 This is in line with recommendations for immunocompromised patient populations in general.2
These recommendations are based on the assumption that this vaccination schedule will lead to better and broader serotype coverage than vaccination with either vaccine alone, that is, a booster effect. This effect has been observed in some immunocompromised patient populations but has not been extensively studied in solid organ transplant recipients. Of note, in a large cohort of liver transplant recipients, vaccination with a conjugate vaccine followed by the polysaccharide vaccine did not lead to a better serologic response than vaccination with the polysaccharide vaccine alone.3 The same was seen in a previous cohort of lung transplant recipients.4 Overall, there have been few studies that investigated different pneumococcal vaccination schedules in solid organ transplant recipients.5,6 In immunocompetent populations, a booster effect has not been observed.7
Ideally, transplant patients should be vaccinated against as many pneumococcal serotypes as possible. Therefore, vaccination with a pneumococcal vaccine that includes the highest number of serotypes (ie, the 23-valent pneumococcal polysaccharide vaccine [23vPPV]) would be preferable, presumed that it can offer the same degree of protection against pneumococcal infections as a conjugate vaccine. An area of concern is serotype-replacement due to mandatory conjugate vaccination in children. This is expected to lead to less pneumococcal infections overall but more pneumococcal infections caused by serotypes that are not included in the conjugate vaccine.8,9
In this study, we investigated the serologic response to pneumococcal conjugate vaccination followed by pneumococcal polysaccharide vaccination in lung transplant candidates and recipients. Our aim was to study a potential booster effect in these specific populations. In addition, we compared vaccination responses in lung transplant recipients with a historical control group of lung transplant recipients who had been vaccinated with the 23vPPV only.
MATERIALS AND METHODS
Patients were recruited from St Antonius Hospital in Nieuwegein, the Netherlands. This hospital is a referral center for lung transplantation in collaboration with University Medical Center Utrecht, the Netherlands. The patient population comprises all types of end-stage lung disease, except for cystic fibrosis. All patients either on the waiting list for transplantation or followed up after transplantation in the period May 2016–January 2019 were included. Patients were given pneumococcal vaccination at the time of placement on the waiting list or, in case of the posttransplantation patients, approximately 5 years after the previous pneumococcal vaccination, independent of the time since transplantation. Patients that were previously vaccinated had all been vaccinated with a 23vPPV only. Usually, this was the case in lung transplant recipients who had already been vaccinated when they were placed on the waiting list. In posttransplantation patients, antipneumococcal antibody levels were followed up for 1 year after vaccination. All patients gave written informed consent for the use of their data in clinical research. The study was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki.
A historical control group that has previously been described10 was used for comparison of the vaccination responses of the lung transplant recipients. This cohort consisted of 55 lung transplant recipients (32 females; median age, 52 y; range 23–60; 31 patients with chronic obstructive pulmonary disease) who were followed up for a median of 6.6 years after transplantation and were vaccinated with a 23vPPV alone.10 These patients had all received 23vPPV before transplantation and were revaccinated approximately 5 years after the first vaccination (median, 4.4 y; interquartile range [IQR], 2.8–6.5 y). The immunosuppressive regimen after transplantation was the same for the present cohort and the historical cohort.
Patients in the present cohort were vaccinated with a 13-valent pneumococcal conjugate vaccine (13vPCV), followed by vaccination with 23vPPV after 8 weeks. Blood samples were drawn before vaccination with 13vPCV, 3 weeks after vaccination with 13vPCV, before vaccination with 23vPPV, and 3 weeks after vaccination with 23vPPV. A blood sample was also taken 1 year after the vaccinations in lung transplant recipients. Serum samples were stored at −80°C until use. Serum IgG antibody concentrations against 13 pneumococcal serotypes were measured using a Luminex platform.11 This included pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F (Danish nomenclature). These are all the serotypes that are included in 13vPCV. All serotypes except for 6A are also included in 23vPPV.
Clinical characteristics, immune status investigations, and follow-up data were collected from patient records. All diagnoses were categorized according to the International Classification of Diseases 10. Bronchiolitis obliterans syndrome (BOS) was defined in accordance with the current international guidelines.12
The 2015 American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma & Immunology classification was used for overall categorization of the antibody response to pneumococcal vaccination.13 A patient is considered to be a normal responder if IgG antibody levels to at least 70% of the serotypes tested are ≥1.3 μg/mL after vaccination, and there is a ≥2-fold increase of postvaccination over prevaccination antibody levels for at least 70% of the serotypes tested. Responses are categorized as (1) normal, (2) mildly impaired (antibody levels ≥1.3 μg/mL for ≥70% of serotypes, but 2-fold or greater increase between prevaccination and postvaccination antibody titers for <70% of serotypes), (3) moderately impaired (antibody levels ≥1.3 μg/mL for <70% of serotypes), or (4) severely impaired (antibody levels ≥1.3 μg/mL for ≤2 serotypes). As we evaluated 13 pneumococcal serotypes, we used a cutoff value of <69% instead of <70%: in case of adequate antibody levels against ≥9 of 13 serotypes, this was categorized as normal.
The immunosuppressive therapy used after lung transplantation consisted of tacrolimus, mycophenolate mofetil, and prednisone. Tacrolimus dosing was based on blood levels. After the first-year posttransplantation, target levels were 7–10 μg/mL. The mycophenolate mofetil dose was 500 mg twice daily, and the prednisone dose 10 mg once daily after the first-year posttransplantation.
For data collection and statistical analyses, SPSS Statistics for Windows (version 24.0) was used. For comparison between 2 groups, the Student t test, Fisher exact test, Mann-Whitney U test, and Wilcoxon signed ranks test were used where appropriate. A p value of <0.05 was considered to be statistically significant. Graphs were designed with GraphPad Prism (version 2.0) and SPSS Statistics for Windows (version 24.0).
RESULTS
Twenty-five lung transplant candidates were included, as well as 23 lung transplant recipients (Table 1). The most common diagnosis in transplant candidates was chronic obstructive pulmonary disease (60% of patients). In transplant recipients, pulmonary fibrosis was more common. Sixty-four percent of the transplant candidates and 83% of the transplant recipients were female, respectively. The median age was 59 years for the lung transplant candidates (IQR, 56–61 y) and 52 years for the lung transplant recipients (IQR, 43–57 y). In transplantation recipients, the median time from transplantation to vaccination was 4.0 years (IQR, 3.1–5.2 y).
TABLE 1.
Baseline characteristics at time of vaccination
Antibody levels for assessing the response to vaccination were available in 24 and 25 of the lung transplant candidates after vaccination with 13vPCV and 23vPPV, respectively. For the lung transplant recipients, antibody levels were available in 23 and 22 patients after vaccination with 13vPCV and 23vPPV, respectively. The median antibody levels significantly increased over baseline for all serotypes after vaccination with 13vPCV in the transplant candidates, as well as in the transplant recipients (Figures 1 and 2).
FIGURE 1.
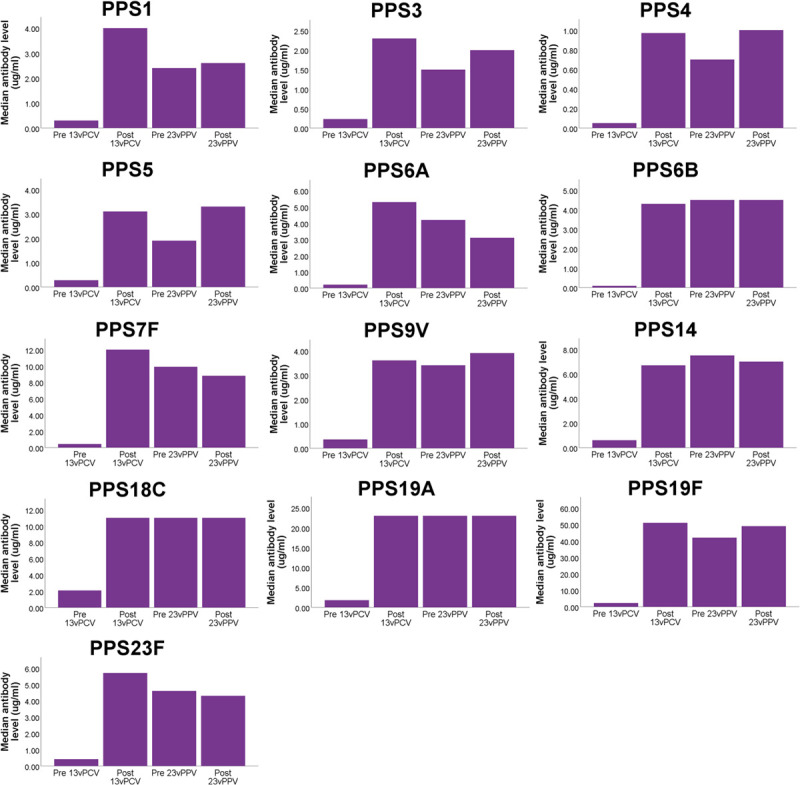
Median serotype-specific antibody levels in lung transplant candidates before vaccination, after vaccination with 13vPCV, before vaccination with 23vPPV (thus 8 wk after 13vPCV), and after vaccination with 23vPPV. Note that the scale of the y-axis is different for all serotypes. Median antibody levels were significantly higher compared with baseline after vaccination with 13vPCV (Wilcoxon signed ranks test; P < 0.01 for all serotypes). 23vPPV, 23-valent pneumococcal polysaccharide vaccine; 13vPCV, 13-valent pneumococcal conjugate vaccine.
FIGURE 2.
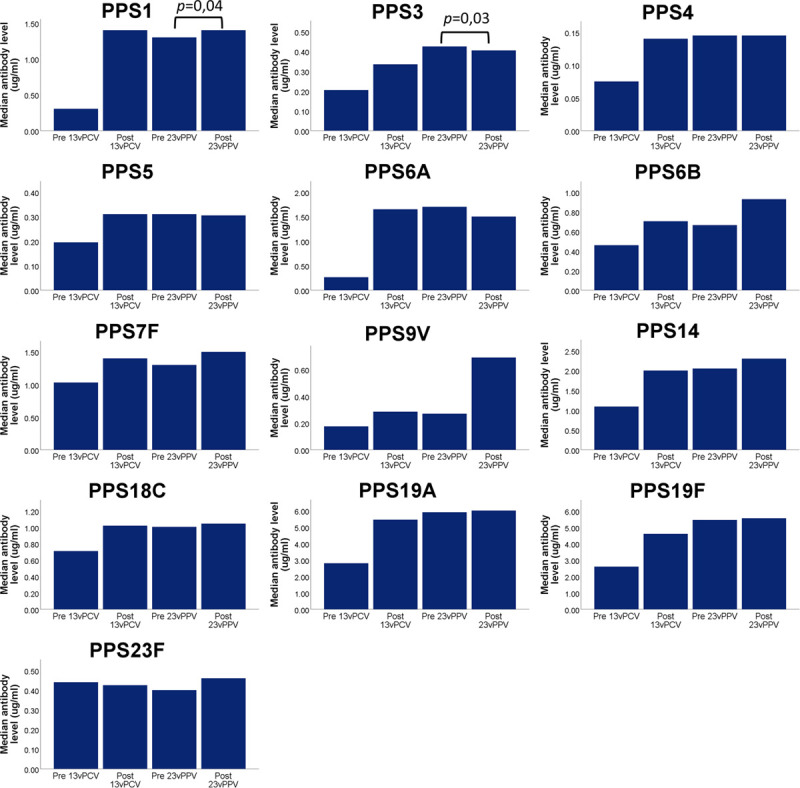
Median serotype-specific antibody levels in lung transplant recipients before vaccination, after vaccination with 13vPCV, before vaccination with 23vPPV (thus 8 wk after 13vPCV), and after vaccination with 23vPPV. Median antibody levels were significantly higher compared with baseline after vaccination with 13vPCV (Wilcoxon signed ranks test; P < 0.01 for all serotypes). The p values shown represent significant differences between antibody levels before 23vPPV (8 wk after 13vPCV) and after 23vPPV (Wilcoxon signed ranks test). 23vPPV, 23-valent pneumococcal polysaccharide vaccine; 13vPCV, 13-valent pneumococcal conjugate vaccine.
Median serotype-specific antibody levels were significantly higher after 23vPPV compared with those before 23vPPV for serotypes 1 and 3 in the lung transplant recipients but not for the other serotypes (Figure 2). In the lung transplant candidates, there was no significant increase in median serotype-specific antibody levels for any serotype after 23vPPV (Figure 1).
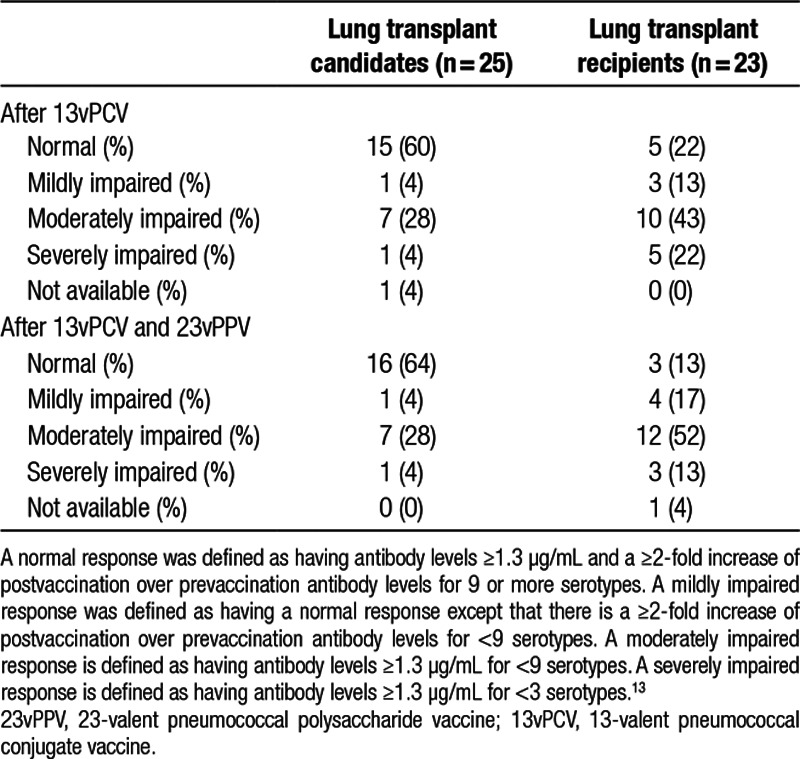
Categorization of the overall response to vaccination is shown in Table 2. In the transplant candidates, 64% of patients could be categorized as a normal responder after vaccination with 13vPCV and 23vPPV. In the transplant recipients, this was 13% of patients. Four percent and 13% of patients in both groups could be categorized as severely impaired responders, respectively. When the response categorization was applied to the response after vaccination with 13vPCV only, this was not significantly different from the response after 13vPCV and 23vPPV.
TABLE 2.
Overall response to pneumococcal vaccination in lung transplant candidates and recipients
Patients were followed up for a median of 1.6 years after vaccination in the transplant candidates (IQR, 1.0–1.9 y) and 1.4 years after vaccination in the transplant recipients (IQR, 0.7–2.0 y). At the end of follow-up, 3 of the transplant candidates had died and 1 had been removed from the waiting list. Five candidates had received a transplant, and the remainder was still on the waiting list. All deceased patients had a normal vaccination response. Of the transplant recipients, 1 patient had died at the end of follow-up; this patient had a severely impaired vaccination response and died due to metastatic renal cancer. Of the transplant recipients, 10 have developed BOS in the course of follow-up (43%). Median follow-up after transplantation was 4.0 years (IQR, 3.1–5.2 y). BOS stages were BOS1 in 5 patients and BOS3 in 5 patients. Development of BOS was directly preceded by an infection in 6 of 10 patients but was not associated with an impaired vaccination response. One patient had recurrent viral, bacterial, and fungal infections and received immunoglobulin replacement therapy after the vaccinations, which led to a markedly reduced infection frequency. This patient had a moderately impaired response to pneumococcal vaccination as well as low IgG and IgG subclass levels. In the course of follow-up, none of the patients had culture-proven pneumococcal infections.
Antibody levels 1 year after vaccination were available for 15 lung transplant recipients. Median serotype-specific antibody levels were significantly lower after 1 year compared with directly after 23vPPV, except for antibodies against serotypes 5, 19F, and 23F (Figure 3).
FIGURE 3.
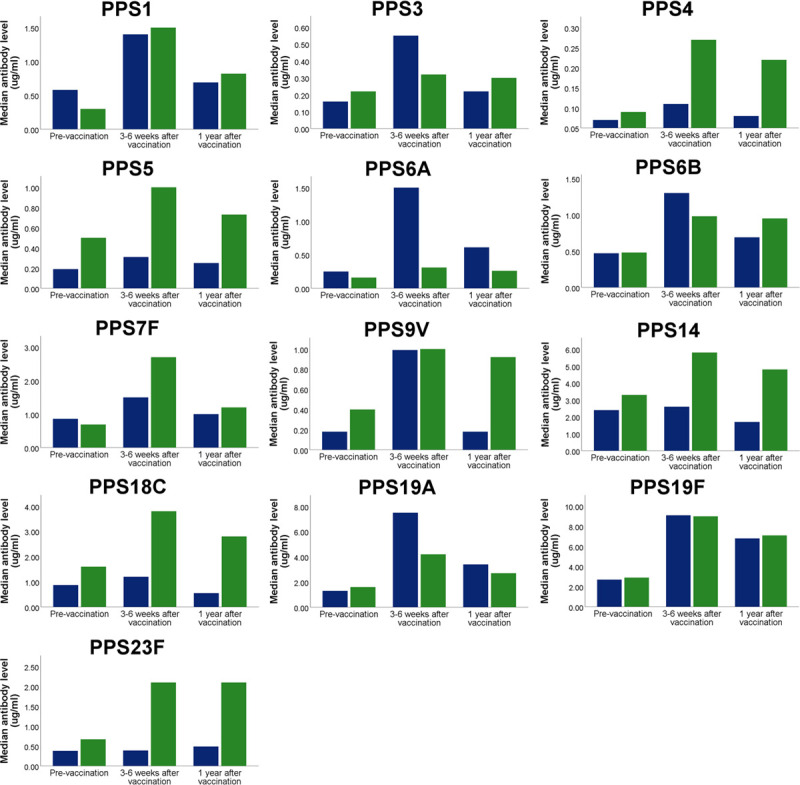
Median antipneumococcal antibody levels in lung transplant recipients. Antibody levels are shown before vaccination, after vaccination (with 23vPPV, ie, after both vaccines in the current study) and 1 y after the vaccinations. Blue bars represent lung transplant recipients from the current study (n = 15), and green bars represent lung transplant recipients from a previously described cohort, who were vaccinated with 23vPPV alone (n = 49). All recipients had been vaccinated with 23vPPV before transplantation as well. 23vPPV, 23-valent pneumococcal polysaccharide vaccine.
When compared with a historical control group of lung transplant recipients vaccinated with 23vPPV only, median serotype-specific antibody levels against serotype 6A were significantly higher 3 weeks after vaccination in the current cohort (P = 0.02; Mann-Whitney U test). The same was seen for fold-increase of serotype-specific antibody levels over baseline (Figure 4). This was significantly higher for serotype 6A in the historical control group (p = 0.01; Mann-Whitney U Test) but not significantly different for other serotypes. Median serotype-specific antibody levels 1 year after vaccination were not significantly different between the 2 groups, except for antibodies against serotype 9 V, which were higher in the control group (P = 0.04; Mann-Whitney U test). The percentage of patients with protective antibody levels (ie, ≥1.3 μg/mL) against a given serotype after 1 year was comparable between the current cohort and the historical control group (Figure 5). When comparing the number of serotypes against which antibody levels were ≥1.3 μg/mL between the current cohort and the historical cohort, there were no significant differences (Figure 6).
FIGURE 4.
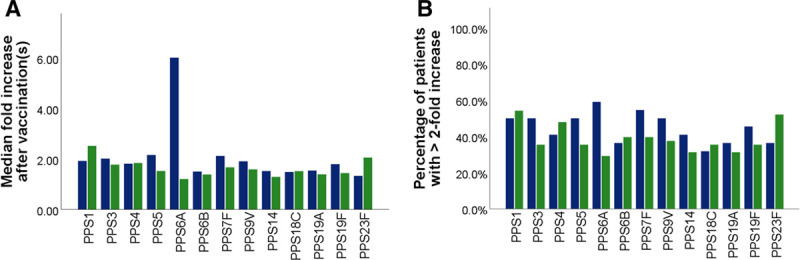
A, Median fold-increase of postvaccination antibody levels over prevaccination antibody levels. Only antibody levels for serotype 6A were significantly different between the 2 groups (higher in current cohort, p = 0.01; Mann-Whitney U Test). B, Percentage of patients with a ≥2-fold-increase of postvaccination antibody levels over prevaccination antibody levels per serotype. Blue bars represent the current cohort, with postvaccination antibody levels measured 3–6 wk after vaccination with 23vPPV (as well as previous vaccination with 13vPCV). Green bars represent the historical control group with postvaccination antibody levels measured 3–6 wk after vaccination with 23vPPV (and no prior vaccination with 13vPCV). 23vPPV, 23-valent pneumococcal polysaccharide vaccine; 13vPCV, 13-valent pneumococcal conjugate vaccine.
FIGURE 5.
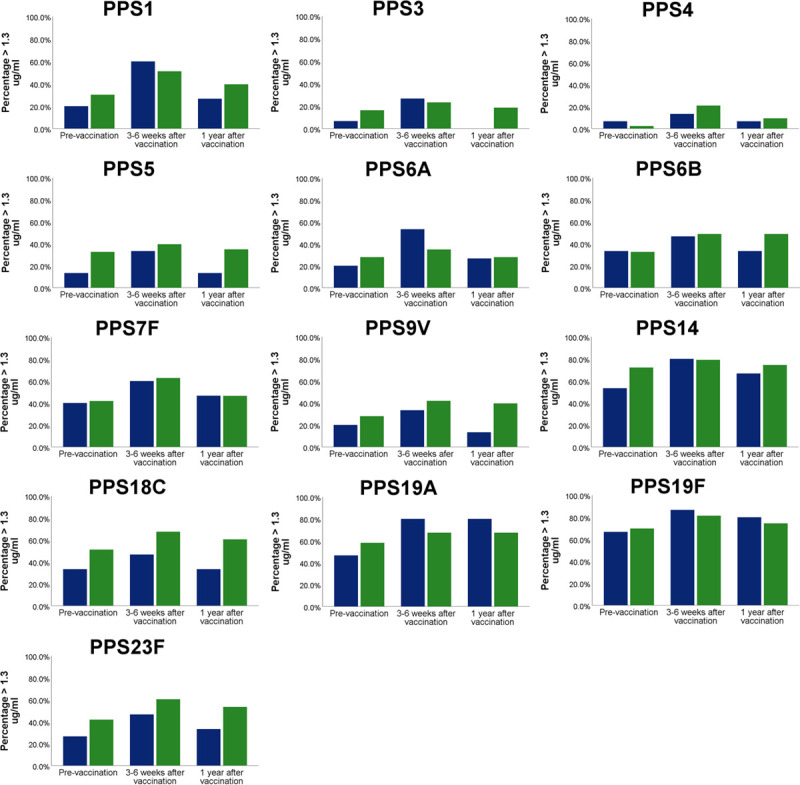
Median percentage of serotypes for which lung transplant recipients have serotype-specific antibody levels ≥1.3 µg/mL before vaccination (with 13vPCV or 23vPPV), after vaccination (with 23vPPV) and 1 y after the vaccinations. Blue bars represent lung transplant recipients from the current study (n = 15), and green bars represent lung transplant recipients from a previously described cohort, who were vaccinated with 23vPPV alone (n = 49). 23vPPV, 23-valent pneumococcal polysaccharide vaccine; 13vPCV, 13-valent pneumococcal conjugate vaccine.
FIGURE 6.
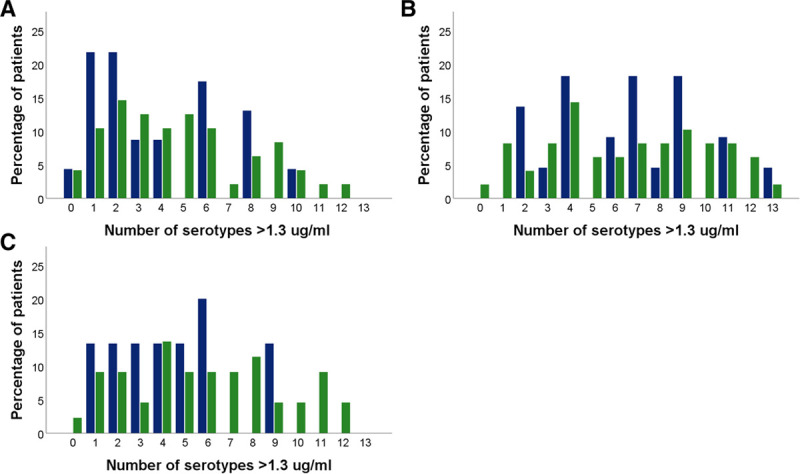
Number of lung transplant recipients with a given number of serotype-specific antibody levels ≥1.3 µg/mL. Blue bars represent lung transplant recipients from the current study (n = 15), and green bars represent lung transplant recipients from a previously described cohort, who were vaccinated with 23vPPV alone (n = 49). (A) before vaccination, median (IQR, 2 [1–8] compared with 5 [3–8]; P = 0.205); (B) after vaccination, in case of the current cohort after vaccination with both vaccines, median (IQR) 7 [4–9] compared with 7 [4–10]; P = 0.975); (C) 1 y after vaccination, median 4 (2–6) compared with 6 (3–8), P = 0.137. IQR, interquartile range; 23vPPV, 23-valent pneumococcal polysaccharide vaccine.
DISCUSSION
In this study, we show that the serologic pneumococcal antibody response after pneumococcal conjugate vaccine did not improve after subsequent vaccination with the 23vPPV in lung transplant candidates and recipients. A booster effect, previously documented in other populations,14–16 was not observed. Also, when compared with lung transplant recipients vaccinated with a polysaccharide vaccine only, vaccination with the conjugate vaccine did not lead to higher antibody levels. Furthermore, follow-up after 1 year in transplant recipients showed a decrease in antibody levels comparable to a historical control group of lung transplant recipients vaccinated with 23vPPV.
An observation that further strengthens the above findings is that the response to pneumococcal serotype 6A did not differ from the response to other pneumococcal serotypes. Specifically, we did not observe a faster or deeper decline in antibody levels against this serotype compared with other serotypes. Serotype 6A is not included in 23vPPV, and therefore no effect on antibody levels would be expected after 23vPPV. A booster effect would not be expected for this serotype, even if it were present for the other serotypes. No significant increase in mean antibody levels to serotype 6A was observed after vaccination with 23vPPV alone in the historical control group, as expected. However, while other serotypes did show a significant increase in median antibody levels in the historical control group, 23vPPV did not lead to a significant increase in median antibody for all serotypes but 1 and 3 when given after 13vPCV.
Our data are in line with previous studies in solid organ transplant recipients that reviewed a vaccination schedule of 13vPCV followed by 23vPPV.3 However, this study is, to our knowledge, the first study of this vaccination schedule in lung transplant recipients. A previous study of heart and lung transplant recipients studied a vaccination schedule of 7vPCV followed by 23vPPV and did not find a booster effect either.4 Overall, there have been few studies that investigated different pneumococcal vaccination schedules in solid organ transplant recipients.5,6 It would seem that especially lung transplant recipients respond poorly to pneumococcal vaccination, which might be related to a relatively high dose of immunosuppressive therapy.17 Waning antibody levels in the year after vaccination have been previously documented in other immunocompromised populations.18,19 This trend was similar in the historic cohort and the present cohort of transplantation recipients and did not seem to be ameliorated by the addition of 13vPCV to 23vPPV.
The current vaccination recommendations for immunocompromised populations are mainly based on research in patients with HIV infection, where a booster effect has been observed. Vaccination with the conjugate vaccine followed by vaccination with the polysaccharide vaccine led to higher antibody levels than vaccination with either vaccine alone or vaccination with the polysaccharide vaccine followed by vaccination with the conjugate vaccine.14,15 This has also been observed in other immunocompromised populations, although extensive studies have not been performed.16 A possible explanation for the lack of a booster effect in lung transplant recipients would be that both T-cell and B-cell immunity are suppressed, as opposed to suppression of mainly T-cell immunity in HIV. In addition, we did not observe a booster effect in lung transplant candidates either, which would be in line with the absence of a booster effect in nonimmunocompromised patient populations.7
One difference between lung transplant recipients and other immunocompromised patient populations is that they receive the immunosuppressive drug tacrolimus, in addition to mycophenolate mofetil and prednisone. Tacrolimus specifically suppresses follicular T-helper cells (in addition to mildly suppressing B-cell function), which are key players in the induction of immunologic memory.20 In general, T cell–dependent antibody responses are thought to be more persistent than T cell–independent responses. As we observed waning antibody levels 1 year after transplantation, this mechanism might be compromised by the use of tacrolimus in transplant recipients. Data on pneumococcal conjugate vaccination responses from patient populations that do not have suppressed follicular T-helper function therefore may not be directly applicable to lung transplant recipients. In a previous study, patients who received tacrolimus monotherapy for rheumatoid arthritis were able to mount a relatively adequate response to 23vPPV, albeit these patients received lower tacrolimus doses than usually given to lung transplant recipients.21 In a cohort of renal transplant patients, maintaining adequate antipneumococcal antibody levels 3 years after 23vPPV was significantly less likely in patients who received tacrolimus instead of cyclosporine A, even though most patients did maintain an adequate response.22 As far as we are aware, there are no data on pneumococcal conjugate vaccine responses in patients receiving tacrolimus monotherapy.
A concern when vaccinating patients with 23vPPV is hyporesponsiveness to subsequent vaccines. This has been seen in various populations, where previous vaccination with 23vPPV could lead to a lower antibody response to subsequent polysaccharide or conjugate vaccines.23 Vaccination with 23vPPV has been shown to lead to a decrease in serotype-specific memory B cells in immunocompetent adults, as well as in asplenic patients.24,25 This was ameliorated by later administration of a pneumococcal conjugate vaccine.24 This effect might be responsible for the lack of benefit of the vaccination schedule with both vaccines over 23vPPV alone in our study. However, the relevance of this phenomenon in lung transplant recipients should be further investigated.
The results suggest that giving both 13vPCV and 23vPPV to lung transplant candidates and recipients, as is recommended by current guidelines, does not provide additional serologic benefits over giving 1 vaccine. If 1 vaccine has to be selected, 23vPPV will be a logical choice, as this provides broader serotype coverage. This is especially relevant in the context of serotype replacement caused by the incorporation of pneumococcal conjugate vaccine into national (childhood) vaccination programs worldwide.9 One concern in this respect is that the epidemiology of pneumococcal serotypes in posttransplantation patients is not well known.26 Previous studies in renal transplant recipient that directly compared 23vPPV to 7vPCV did not show a benefit of one vaccine over the other but did show that antibody levels declined after several years in both groups.18,27,28
Another potential benefit of using 23vPPV is that measurement of the polysaccharide antibody response to this vaccination can give additional information about the humoral immune status of the patient. An impaired response could have therapeutic consequences such as starting immunoglobulin replacement therapy.
There are several limitations to this study. First, as is the case in most studies in solid organ transplant recipients, this was a relatively small cohort. This prohibits the studying of “hard” endpoints such as pneumococcal infections and mortality because of the small sample size. During the follow-up, only 1 of the lung transplant recipients died, which makes any associative analyses impossible. Second, we only studied quantitative antibody responses and not antibody avidity. The latter is also important for the effectiveness of the antibody response. Third, we used the diagnostic criteria for specific antibody deficiency as a measure of immune function, but these criteria are subject to debate. In these criteria, the same cutoff level is used for all pneumococcal serotypes, but it is likely that using serotype-specific cutoff values would give a more precise indication of the functioning of the immune system.17,29 In addition, the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma & Immunology diagnostic criteria13 have never been validated in immunocompromised patient populations. Fourth, in the past, a different assay to quantify antipneumococcal antibodies was used. Therefore, the comparison of absolute values between the present cohort and the historical cohort needs to be interpreted with caution. However, we mainly used fold-increase values and comparison of antibody levels to prespecified cutoff values in interpreting the results. These methods are in line with the use of pneumococcal antibody responses to diagnose an antibody deficiency.13 This is an internationally acknowledged guideline that is used for different antibody assays in different laboratories. We have not been able to do a bridging analysis, as the original assay form was no longer available.
In conclusion, serologic vaccination responses in lung transplant candidates and recipients were not improved by giving 23vPPV after 13-valent pneumococcal conjugate vaccine. We did not observe a booster effect. All transplant recipients had also received a polysaccharide vaccine before transplantation. When compared with historical controls vaccinated with the polysaccharide vaccine only, vaccination with the conjugate vaccine did not lead to higher antibody levels. It remains to be determined what the optimal pneumococcal vaccination schedule is for lung transplant candidates and recipients.
ACKNOWLEDGMENTS
We thank Ms M. Langezaal, Ms M. de Wit, Ms H. Snijders-Tijink, and Ms M. Jansen-Hagen for their assistance with vaccination scheduling and administration and Ms H. van Velzen-Blad for her insightful comments on an earlier version of this article. We thank the laboratory technicians for their assistance with the measurement of the pneumococcal antibody levels.
Footnotes
Published online 21 May, 2020.
The authors declare no funding or conflicts of interest.
T.W.H. participated in data collection, data analysis, interpretation of the results, and wrote the first draft of the article. B.M. was responsible for the measurement of antipneumococcal antibodies and participated in data analysis, interpretation of the results, and commented on the first draft of the article. G.T.R. and J.C.G. participated in the interpretation of the results and commented on the first draft of the article. D.A.v.K. designed the study, participated in data analysis, interpretation of the results, and wrote the first draft of the article. All authors agree with the submitted version of the article.
REFERENCES
- 1.Danziger-Isakov L, Kumar D; AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13563. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep. 2012;61:816–819. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6140a4.htm. Accessed February 16, 2020. [PubMed] [Google Scholar]
- 3.Kumar D, Chen MH, Wong G, et al. A randomized, double-blind, placebo-controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in adult liver transplant recipients. Clin Infect Dis. 2008;47:885–892. [DOI] [PubMed] [Google Scholar]
- 4.Gattringer R, Winkler H, Roedler S, et al. Immunogenicity of a combined schedule of 7-valent pneumococcal conjugate vaccine followed by a 23-valent polysaccharide vaccine in adult recipients of heart or lung transplants. Transpl Infect Dis. 2011;13:540–544. [DOI] [PubMed] [Google Scholar]
- 5.Kim YJ, Kim SI. Vaccination strategies in patients with solid organ transplant: evidences and future perspectives. Clin Exp Vaccine Res. 2016;5:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dendle C, Stuart RL, Mulley WR, et al. Pneumococcal vaccination in adult solid organ transplant recipients: a review of current evidence. Vaccine. 2018;36:6253–6261. [DOI] [PubMed] [Google Scholar]
- 7.Goldblatt D, Southern J, Andrews N, et al. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis. 2009;49:1318–1325. [DOI] [PubMed] [Google Scholar]
- 8.Wagenvoort GHJ, Sanders EA, Vlaminckx BJ, et al. Invasive pneumococcal disease: clinical outcomes and patient characteristics 2-6 years after introduction of 7-valent pneumococcal conjugate vaccine compared to the pre-vaccine period, the Netherlands. Vaccine. 2016;34:1077–1085. [DOI] [PubMed] [Google Scholar]
- 9.Musher DM, Rodriguez-Barradas MB. Why the recent ACIP recommendations regarding conjugate pneumococcal vaccine in adults may be irrelevant. Hum Vaccin Immunother. 2016;12:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kessel DA, Hoffman TW, Kwakkel-van Erp JM, et al. Long-term follow-up of humoral immune status in adult lung transplant recipients. Transplantation. 2017;101:2477–2483. [DOI] [PubMed] [Google Scholar]
- 11.Elberse KE, Tcherniaeva I, Berbers GA, et al. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol. 2010;17:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. [DOI] [PubMed] [Google Scholar]
- 13.Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2014;136:1186–1205.e1–e78. [DOI] [PubMed] [Google Scholar]
- 14.Kroon FP, van Dissel JT, Ravensbergen E, et al. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine. 2000;19:886–894. [DOI] [PubMed] [Google Scholar]
- 15.Lesprit P, Pédrono G, Molina JM, et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS. 2007;21:2425–2434. [DOI] [PubMed] [Google Scholar]
- 16.Chan CY, Molrine DC, George S, et al. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin’s disease. J Infect Dis. 1996;173:256–258. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman TW, van Kessel DA, Rijkers GT. Impact of using different response criteria for pneumococcal polysaccharide vaccination for assessment of humoral immune status. J Clin Immunol. 2018;38:149–152. [DOI] [PubMed] [Google Scholar]
- 18.Kumar D, Welsh B, Siegal D, et al. Immunogenicity of pneumococcal vaccine in renal transplant recipients—three year follow-up of a randomized trial. Am J Transplant. 2007;7:633–638. [DOI] [PubMed] [Google Scholar]
- 19.Papadatou I, Lagousi T, Kattamis A, et al. Antibody persistence 5 years after a 13-valent pneumococcal conjugate vaccine in asplenic patients with β-thalassemia: assessing the need for booster. Ann Hematol. 2019;98:775–779. [DOI] [PubMed] [Google Scholar]
- 20.Wallin EF, Hill DL, Linterman MA, et al. The calcineurin inhibitor tacrolimus specifically suppresses human T follicular helper cells. Front Immunol. 2018;9:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migita K, Akeda Y, Akazawa M, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tacrolimus. Arthritis Res Ther. 2015;17:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindemann M, Heinemann FM, Horn PA, et al. Immunity to pneumococcal antigens in kidney transplant recipients. Transplantation. 2010;90:1463–1467. [DOI] [PubMed] [Google Scholar]
- 23.Papadatou I, Spoulou V. Pneumococcal vaccination in high-risk individuals: are we doing it right? Clin Vaccine Immunol. 2016;23:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clutterbuck EA, Lazarus R, Yu LM, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papadatou I, Orthopoulos G, Theodoridou M, et al. Long-lasting hyporesponsivenss induced by the 23-valent pneumococcal polysaccharide vaccine (PPV23) in asplenic patients with β-thalassemia major. Vaccine. 2015;33:3779–3783. [DOI] [PubMed] [Google Scholar]
- 26.Kumar D, Humar A, Plevneshi A, et al. Invasive pneumococcal disease in solid organ transplant recipients—10-year prospective population surveillance. Am J Transplant. 2007;7:1209–1214. [DOI] [PubMed] [Google Scholar]
- 27.Kumar D, Rotstein C, Miyata G, et al. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis. 2003;187:1639–1645. [DOI] [PubMed] [Google Scholar]
- 28.Tobudic S, Plunger V, Sunder-Plassmann G, et al. Randomized, single blind, controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in renal transplant recipients. PLoS One. 2012;7:e46133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaballie H, Bosch B, Schrijvers R, et al. Fifth percentile cutoff values for antipneumococcal polysaccharide and anti-Salmonella typhi Vi IgG describe a normal polysaccharide response. Front Immunol. 2017;8:546. [DOI] [PMC free article] [PubMed] [Google Scholar]


