Abstract
“Brittle diabetes” was first used to describe a life “disrupted by episodes of hypoglycemia or hyperglycemia.” Early descriptions focused on small case reports of mostly young women with psycho-social instability, recurrent diabetic ketoacidosis, poor patient compliance or maladaptation. We redefine “brittle diabetes” as occurring in four specific life stages each with distinct characteristics and associated conditions resulting in severely erratic glycemic control and poor outcomes. Once identified however these factors can often be reversed or significantly mitigated.
The first group includes younger patients with associated psychiatric diseases such as bulimia and depression which require specific therapy and are treatable. A second group includes individuals who have another underlying medical condition resulting in disruption of insulin sensitivity or glucose utilization which must be sought. A third group, the largest we believe, has “geriatric type 1 diabetes” and develops severe glycemic instability due to frailty, chronic renal failure, dementia, vision loss, loss of counterregulation and other diseases of aging which lead to unintentional omission of insulin, insulin dosing errors and increasing insulin sensitivity. The fourth group, now seen around the world, suffers lack of insulin access and associated food insecurity. All four of these groups are described.
Keywords: Brittle diabetes, Diabetic ketoacidosis, Hypoglycemia, Unstable diabetes, Labile diabetes
1. Introduction
The term “brittle diabetes” was first described by Woodyatt in 1934 to describe individuals with large, unexplained changes in blood glucose concentrations.1 Over the years the definition evolved to characterize diabetes in a patient whose “life was constantly disrupted by episodes of hypoglycemia or hyperglycemia”.2 There have been many other subsequent definitions of brittle diabetes noting lability of glycemia, frequent hospitalizations for diabetic ketoacidosis (DKA) or hypoglycemia (Table 1 ), and often poor patient outcomes. In 1985 Schade and colleagues focused on the etiologies of severe disruptive glucose lability and noted that in 30 affected patients, 16 were found to have “fictious disease” or “malingering”.3 The term thus took on tones of a largely maladaptive patient coping response.
Table 1.
Etiologies of brittle diabetes.
Generally resulting in hyperglycemia/ketoacidosis
Generally resulting in hypoglycemia
Generally resulting in either hyperglycemia or hypoglycemia
|
Brittle diabetes is still frequently noted by clinicians in a variety of clinical outpatient settings and often is included in hospital admission and discharge diagnoses, yet is actually a descriptive term and is not included in the classification of diabetes from either the American Diabetes Association or the World Health Organization. It is also not a form of “atypical diabetes” as most individuals with brittle diabetes have either type 1 diabetes or severely insulin deficient type 2 diabetes.
Clearly, as our medical knowledge has expanded, the old descriptive terminology is outdated. While it is accurate to consider these patients as having life-changing disruptions due to severe glycemic dysregulation, classifying them as “brittle” may feel condescending to many patients and their families. Furthermore, the term can convey a sense of patient blame or maladjustment which may be either inaccurate or incomplete and blunts a more appropriate impetus to search for treatable causes of this life-threatening condition and appropriate interventions to remedy it.
In fact, most patients who have extreme difficulty controlling their diabetes have other conditions (sometimes undiagnosed) which may include mental health disorders, significant psycho-social stressors, a coexisting medical problem or factors of age, transitions and socioeconomic constraint. Thus, unstable diabetes control may be a primary or secondary condition. Whatever the causal factors these patients are at risk for many unfortunate consequences: poor glycemic control with its complications, more emergency room and hospital admissions, strains on relationships, and greater healthcare utilization and diabetes distress.
We find it helpful to separate these patients with “brittle diabetes” into four groups which tend to cluster according to lifespan to some degree. The first group, as described by Schade et al.,3 are often younger woman who are DKA-prone and have severe glycemic variability usually due to psycho-social instability and/or significant psychiatric disease and/or some form of an eating disorder which is often not being addressed. The second group would be those individuals who have another medical condition resulting in a major disruption of insulin sensitivity/utilization or a nutritional disease-causing insulin/glucose mismatching. Identifying and treating co-existing morbidity can often resolve the lability of glycemia completely. The third group has emerged as individuals with type 1 diabetes now living into older age and suffering various degrees of cognitive impairment and life-style transition resulting in inability to self-manage their diabetes. “Geriatric type 1 diabetes” was rarely seen 30 years ago but with the longer life spans of these patients today we frequently now see severe glycemic instability due to unintentional omission of insulin doses, confusion of insulin types and insulin amounts, dosing errors or repeated insulin doses. Furthermore this population can experience failures to accurately utilize self-glucose monitoring technologies and, due to coexisting impaired glucoregulatory responses, become highly prone to severe hypoglycemic emergencies. A final condition which until recently was mostly seen in low-income countries but now is seen around the world, is lack of insulin access (in the United States due to cost) and food insecurity. These patients also meet the definition of brittle diabetes due to the severe glycemic variability and incapacitation they experience repeatedly to the point of DKA. Each of these groups will be described.
2. DKA/hypoglycemia-prone due to psychological disease
These are the patients similar to those first described with brittle diabetes in the classic report by Schade et al.3 Of the 16 patients described, 14 were reported to have either “factitious disease” or “malingering” and were most often young women ranging in age from 8 to 33 years. In the Tattersall report, there was no difference in gender for those with recurrent DKA, but these investigators also noted a group with recurrent severe hypoglycemia.2 Indeed, they described a group called “mixed brittleness” with recurrent DKA and severe hypoglycemia. While psycho-social stressors were usually the etiology of these hospital admissions, it is difficult to know how many in the severe hypoglycemia group had developed hypoglycemia unawareness which is better understood and treated today.4
Adolescence and young adulthood are particularly challenging times for those with type 1 diabetes. Indeed, in the T1D Exchange the mean A1C for those 13 to 25 years of age was 9.3% and for those not using continuous glucose monitoring (CGM) or insulin pump therapy was 9.6%.5 Between 4 and 6% of these individuals had at least one episode of DKA or severe hypoglycemia during the 3 months before the survey. It has long been acknowledged that this age group often struggles with adherence to prescribed insulin regimens6 as they transition from childhood care to the requirements of selfcare in adolescence and young adulthood. Indeed, the more complex regimens for T1D today are vastly different from the one to two injections of previous eras. By definition, this group meets the criteria for brittle diabetes. In the Schade report, 18 of the 30 patients were in this age group. Thus, Voulgari notes when this population reports taking insulin doses of over 2.0 units/kg/day, medication non-adherence needs to be considered.6 However, there are still other reasons for severe insulin resistance in young type 1 diabetes. Puberty, chronic infection, steroid administration, hormonal contraceptives, and menstrual fluctuations are obvious examples. There are also age-related causes of hypoglycemia including “dieting”, irregular food intake, fear of weight gain, transitions from family eating to self-provided meals, intensive exercise with sports participation, recreational activities, drugs and alcohol use and occult eating disorders common in those with and without diabetes in the adolescent and young adult population.
Severe mental health problems contribute to brittle diabetes in all age groups.7 , 8 Family dysfunction, eating disorders, and factious disease (particularly among health care professionals) and associated addictions to alcohol and drugs have all been described.9 , 10 The primary diabetes provider must have a high index of suspicion for these diagnoses, (particularly eating disorders and factitious disease), and once a diagnosis is suspected a prompt referral should be made to an experienced psychiatrist, psychologist, or medical social worker familiar with the interaction of mental health and acute and chronic diabetes management.
It is important to note that most reports acknowledge the profound psychosocial problems that challenge all patients with diabetes. There was an assessment comparing 42 subjects with brittle diabetes (“severe life-disrupting glycemic instability of any kind”) and a case control group of 42 subjects with “stable diabetes” matched for age, gender, years of education, and diabetes duration. Surprisingly, there were no differences noted in all primary symptom's dimensions and in the three global distress indices between the two groups.11 Those with brittle diabetes did show higher percentages of borderline, histrionic, and narcissistic personality disorders however, these patients were older than those described in the Tattersall2 and Schade3 reports.
Since it is difficult to define what constitutes a relatively subjective definition of “life constantly disrupted by episodes of hypoglycemia or hyperglycemia,” it is important to review epidemiologic data about repetitive DKA. In one report of 24,890 pediatric DKA admissions between 2004 and 2006, 20.3% were readmissions within one year of the first.12 How many of these would be considered to have “brittle diabetes”? It would be ideal to have a more objective definition of this diagnosis to identify those who could benefit from referral for appropriate psychological therapy.
Not surprisingly, the cost of brittle diabetes resulting in DKA is not trivial. In a report from 2013 in the US the mean hospital cost for a pediatric DKA admission was over $7100.12 It is more difficult to obtain cost data for hypoglycemia for this age group, but in an older population of insulin users the annualized medical cost of hypoglycemia by itself (emergency rooms and hospital admissions) was $3241.13 Another report specifically addressing brittle diabetes noted costs were almost three-fold times the yearly costs for stable diabetes management.14 It is clear the financial cost of acute metabolic emergencies common in brittle diabetes is expensive.
Treatment of these patients can be challenging and frustrating. Each case is different and requires creative strategies.3 , 10 Most important is referral to appropriate mental health providers, ideally knowledgeable in diabetes. In our experience this often needs to include the entire family. In previous eras these patients were admitted to the hospital for therapy, but that is not generally realistic now. There are reports of good results in the outpatient setting.15
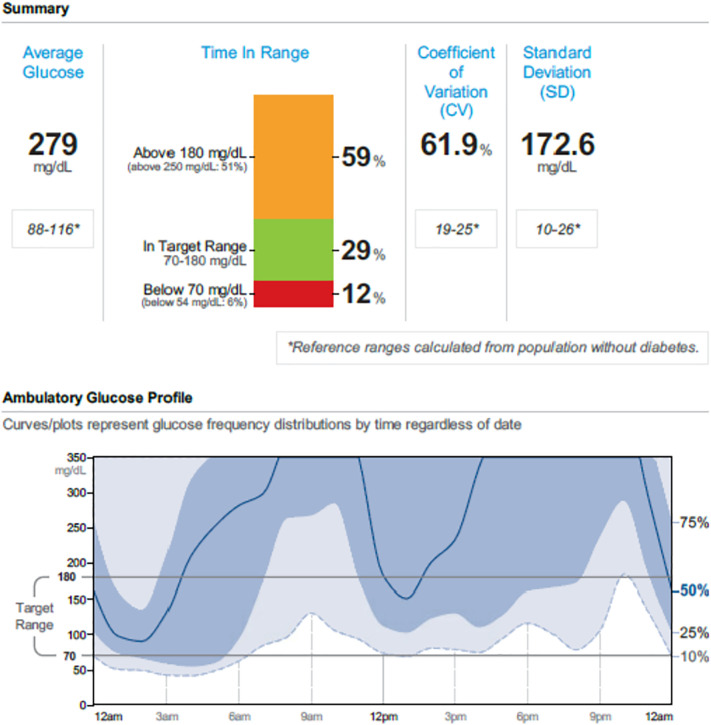
As an example, a 24-year-old woman was referred for “brittle diabetes”. She developed her diabetes at the age of 19 years and had been hospitalized at her community hospital on average 4 to 5 times yearly for DKA. Her reported insulin regimen included 500 units of insulin degludec once daily and 150 to 200 units of insulin lispro before meals (15 units/kg/day). She worked as a clerk in a pharmacy. On her first visit (she took her insulin degludec that morning and her lispro two hours before the visit), a random blood draw showed she was in mild DKA with her plasma glucose of 623 mg/dL, bicarbonate of 19 mEq/L, and anion gap of 16. Her total insulin measured at the same time as was 0.9 μU/mL and her free insulin was less than 1 μU/mL. Her insulin antibodies were negative. Her CGM is shown in Fig. 1 .
Fig. 1.
Continuous glucose monitoring download for 30 days of a patient with brittle diabetes.
She came back fasting and reported that she had taken her usual dose of insulin degludec and 135 units of insulin lispro for hyperglycemia 30 min prior to the appointment. After a blood draw, she was administered (from a new vial of insulin) 10 units of insulin lispro. Her plasma insulin and glucose levels are noted in Table 2 .
Table 2.
Insulin and glucose levels after 10 units of insulin lispro in a 24 year-old woman with brittle diabetes.
| Time (hour) | Insulin (μU/mL) | Glucose (mg/dL) |
|---|---|---|
| 0 | 1.2 | 524 |
| 1 | 49.2 | 346 |
| 2 | 29.5 | 142 |
| 3 | 17.8 | 125 |
Her low insulin levels at baseline with a robust increase during the next few hours along with a normal reduction in blood glucose and her CGM download, were proof of factitious and intermittent insulin administration. Reviewing these findings with the patient can be challenging and in our experience is best performed with family psychological support and members present. The reasons for this patient's behavior were not clear, and if it meets the criteria of Munchausen's Syndrome the diagnosis ideally is made by a mental health provider. Denial can be common despite this type of strong proof. Ideally, patients like this should receive psychological assistance from a knowledgeable professional experienced with addressing this type of self-destructive behavior.
We have also seen poor glycemic control meeting the definition of brittle diabetes with severely depressed patients at all ages. After appropriate treatment with psychotherapy and pharmacotherapy, the diabetes self-management can improve, although the literature on this point is mixed.16
3. Severe glucose variability due to co-existing medical condition
There are numerous reasons for co-existing medical conditions to result in brittle diabetes. (Table 1). It is important to appreciate however that these are generally uncommon. For example, hyperglycemia and DKA can be caused by counterregulatory hormone hypersecretion, although this rarely results in such severe instability that the definition of brittle diabetes is achieved. Nevertheless, there are reports of thyrotoxicosis, acromegaly, Cushing's Syndrome glucagonoma, and pheochromocytoma all associated with DKA.17., 18., 19., 20., 21. While infection is a well-recognized etiology of insulin resistance (including COVID-19),22 there are no reports of infection resulting in brittle diabetes. One of us (IBH) cared for a patient with cystic fibrosis-related diabetes who became severely insulin resistant to the point he required U-500 insulin. While receiving this concentrated insulin he had two episodes of hypoglycemic seizures in addition to frequent post-prandial blood glucose levels above 400 mg/dL. After a year of receiving the U-500 insulin, he was diagnosed with central nervous system norcardiosis. After infection treatment, his insulin sensitivity returned to his baseline and the concentrated insulin was discontinued. Another patient seen by one of us (LMG) treated an older non-obese man with type 2 diabetes requiring over 200 units per day of insulin and multiple hospitalizations for impending DKA. Ultimately, he was found to have an infected hip and after joint replacement his glycemic control improved and his insulin requirements were a fraction of what they had been.
There are genetic etiologies of insulin resistance resulting in brittle diabetes. Rare congenital lipodystrophies (Berardinelli-Seip congenital lipodystrophy) and familial partial lipodystrophy, (Kobberling-Dunnigan Syndrome) in addition to mitochondrial diabetes are often listed as etiologies of brittle diabetes but there are no case reports where the current definition is met. Other rare syndromes which have been described to result in brittle diabetes include a case of Stiff Man (Person) Syndrome which caused brittle diabetes and DKA of unknown etiology.23
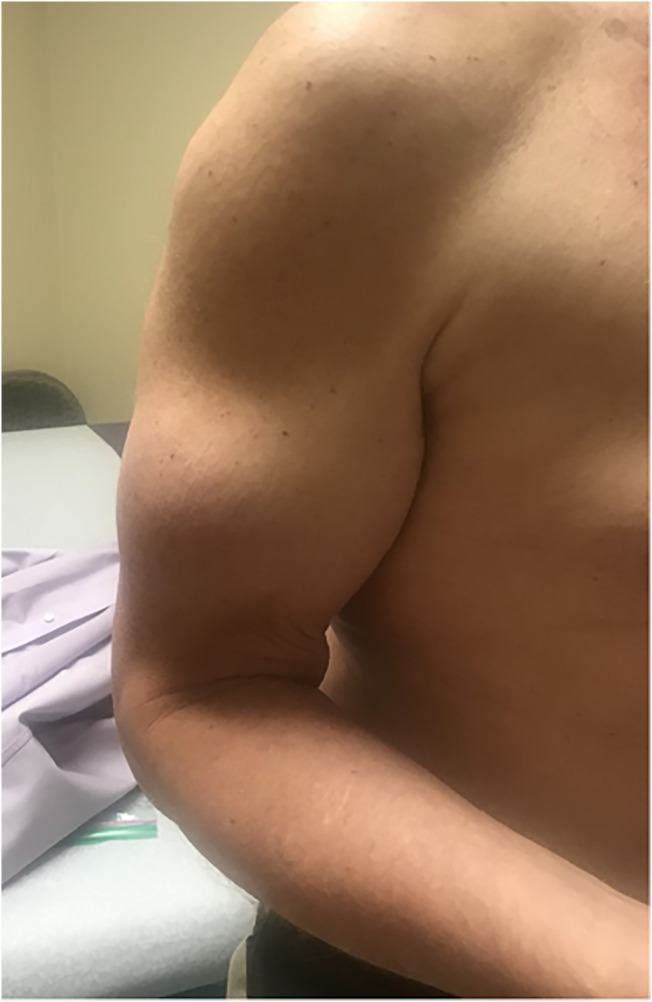
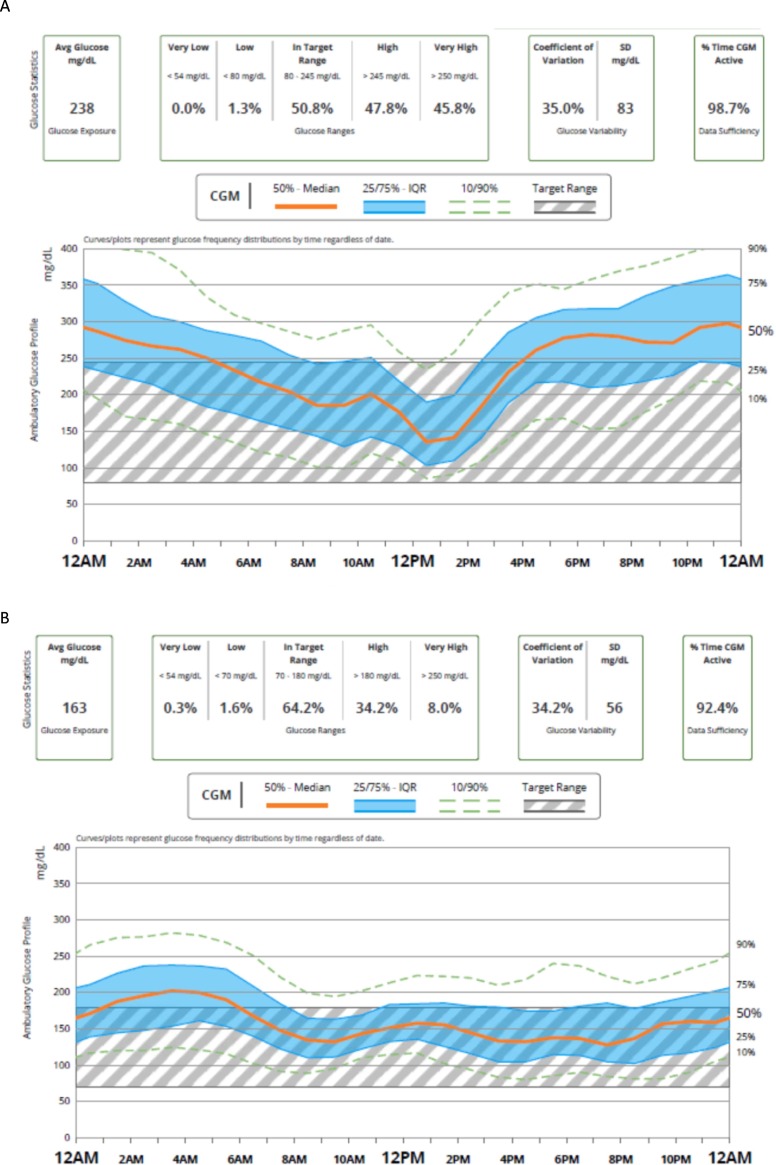
A more common etiology for brittle diabetes is lipohypertrophy from repeated insulin injections in the same site (Fig. 2 ). Insulin injected into lipohypertrophic tissue can cause erratic insulin absorption resulting in fluctuating glucose levels resulting in both hypo- and hyperglycemia.24 As seen in Fig. 3 , simply injecting in new sites can make dramatic changes in blood glucose control.
Fig. 2.
Lipohypertrophy in an arm from decades of repeated insulin injections.
Fig. 3.
A. CGM from a patient with type 1 diabetes receiving insulin degludec and aspart into areas of lipohypertrophy. B. CGM one year later injecting into different injection sites.
In terms of hypoglycemia, one etiology for brittle diabetes is diabetic gastroparesis. In the T1D Exchange, almost 5% of the population (mean age 49 years) was diagnosed with gastroparesis.25 Severe hypoglycemia, defined as seizure or coma, occurred one or more times per year in 25% of those with gastroparesis compared to 11% of those without.25 Another cause of life-threatening hypoglycemia is the type B insulin resistance syndrome.26 This condition is classically seen in middle-aged black women with severe insulin resistance and intermittent hypoglycemia.
There are other cited etiologies for brittle diabetes that are not supported by the evidence. For example, pancreatic diabetes from pancreatectomy is not associated with brittle diabetes. One would think that the glucagon deficiency seen in this most severe form of pancreatic diabetes would result in more glycemic lability and hypoglycemia. In fact, there are no differences in diabetes-specific outcomes after pancreatectomy compared to type 1 diabetes.27 Alcohol is another etiology frequently noted as causing brittle diabetes due to hypoglycemia. While it has been appreciated for decades that alcohol suppresses hepatic gluconeogenesis increasing hypoglycemia risk,28 severe hypoglycemia does not frequently appear to be a sequala of alcohol intoxication.29
4. Geriatric type 1 diabetes
The number of older adults with type 1 diabetes continues to increase. There are two reasons for this. First, life expectancy of those with type 1 diabetes continues to improve.30 Even prior to the development of human insulin and its analogues, home blood glucose monitoring, insulin pumps, and certainly CGM, life expectancy improved to 69 years if diagnosis occurred between 1965 and 1980.31 In addition, we are now seeing an increasing number of people with type 1 diabetes diagnosed in adulthood and surviving to advanced ages. One recent study showed 42% of newly diagnosed type 1 diabetes occurred between the ages of 31–60 years.32 It is not uncommon to see grandparents of children with type 1 diabetes develop the condition. Prime Minister Theresa May developed her type 1 diabetes at the age of 57 years,33 while comedian Jerry Lewis noted at the age of 90 that he had developed his type 1 diabetes late in his life.34
Type 1 diabetes can be difficult at any age, but self-management for elderly patients can be particularly challenging. Concerns with remembering to administer insulin (or taking it twice) are a common problem that can result in enough disruption to meet the criteria of brittle diabetes. As cognition declins with age, independent self-care of diabetes can become problematic for many. Often emergency department admissions for an acute glycemic emergency require family members to become more involved in their elder's daily care. Inexperienced family members, care givers and even skilled nursing facility staff often find management of type 1 diabetes difficult and they also make insulin errors. Mismatches of nutrition with insulin in terms both of dosing and timing are extremely common and a major cause of recurrent emergency room and hospital admissions in this group.
Hypoglycemia is a major concern as in this age group as it is associated with more cardiovascular events and serious health outcomes.35 Using blinded CGM, older adults with type 1 diabetes (mean age 68 years) were found to be hypoglycemic, below 70 mg/dL and 50 mg/dL, for a median of 91 and 32 min each day, respectively. Severe sequelae of both short-term and long-term effects of hypoglycemia have led to newer guidelines for this age group and advise avoidance of hypoglycemia by increasing glycemic targets and up-adjusting alarm settings.36
Fortunately, CGM has been shown to be efficacious in this age group when used properly and in the United States CGM is covered by Medicare for insulin deficient patients meeting certain criteria. In the Wireless Innovation for Seniors with Diabetes Mellitus (WISDM) study, 203 individuals with a median age of 68 years showed that when using real-time CGM hypoglycemic burden (below 70 mg/dL) was reduced by 27 min per day.37 Importantly, use of CGM was also found to result in less hypoglycemia below 54 mg/dL and less severe hypoglycemia (requiring the assistance of another person). Finally, while there is no consensus as to what degree of glycemic variability is ideal, let alone what degree results in brittle diabetes in the aged, those individuals using CGM had a coefficient of variation (CV) of 37% compared to 42% for those using blood glucose monitoring.
It needs to be emphasized that despite the plethora of new tools, older patients with type 1 diabetes face specific challenges. Cognitive losses of all degrees, challenges with dexterity, loss of vision and hearing, frequent depression often due to the loss of a spouse/partner or other family members, chemotherapy regimens, and many other co-morbidities can lead to frequent problems with day-to-day diabetes management. Skilled nursing facilities are often not equipped or prepared to manage individuals with such high daily medical needs. Given the explosion in the number of seniors with type 1 diabetes we will be seeing in the next decade, greater effort is required in preparing providers and our health care systems in managing “brittle diabetes” in this population.
5. Insulin and food insecurity
From a global viewpoint, lack of insulin access in low-income countries is a major cause of pediatric mortality.38 Technically, this could be considered brittle diabetes since it meets the definition of constant disruption of life, in this case due to hyperglycemia and DKA. While this is not what the Canadian discoverers of insulin envisioned almost 100 years ago, they would be even more appalled by the fact that insulin access and “insecurity” are now increasingly common and problematic in more affluent countries as well.38,39 A 2018 report from the non-profit T1International noted 26% of respondents from the United States acknowledged insulin rationing at least once in the previous year.40 Deaths from insulin rationing are not uncommon even in this high-income country,41 and it is unclear if the politicians will be able to rectify the problem. While the original descriptions of brittle diabetes did not include insulin insecurity,1., 2., 3., 4. it unfortunately must be considered a cause today and an increasing challenge for both those in low-income and high-income nations globally.
Food insecurity refers to the uncertain or limited access to adequate and safe foods and has had minimal attention compared to access to insulin. Not surprisingly, this is problematic in low-income countries42 but also in low-income communities in the US.43 Food insecurity is associated with higher A1C levels in both type 1 and type 2 diabetes.43 , 44,45 For children whose parents are food insecure, there is a three-fold increase in yearly hospitalization compared to food secure families (30% vs. 10.5%, p = .002).44 From this report, it isn't clear if this is for DKA or hypoglycemia, but given that high a prevalence, most would agree that this meets the definition of brittle diabetes. Food and medication insecurity often co-exist,46 so a child or adult with type 1 diabetes in this situation is particularly challenged.
Since this is not a typical screening topic for clinicians, for many reasons (including time required) it is difficult to intervene for the most vulnerable patients. Still, for many parents of children with diabetes, having the ability to discuss this sensitive topic is appreciated.47 For others, the stigma and judgement by clinicians make it difficult to have an honest discussion. This is an area that needs more effort and research as to how it contributes to brittle diabetes and how it can be prevented.
6. Conclusions
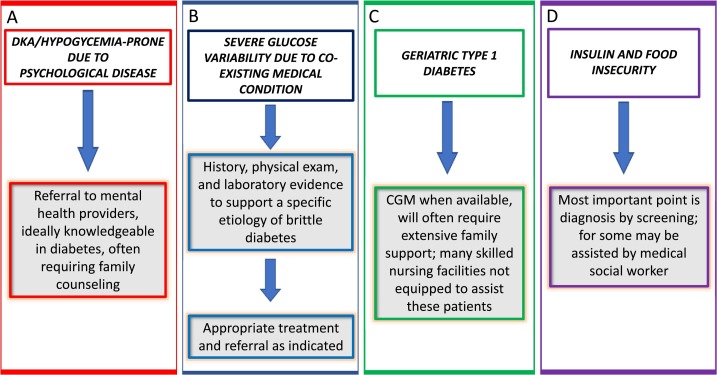
Physicians have been classifying diabetes as “brittle” for decades to indicate a condition of instability of glycemic control requiring frequent hospitalizations and causing impaired quality of life and economic cost. The true prevalence of this diagnosis by the definition of “life constantly disrupted by episodes of hypoglycemia or hyperglycemia” is unknown but many clinicians see patients like this. It is interesting to note that in the past 20 years, there are only 198 PubMed references about “brittle diabetes” suggesting this is not a common clinical challenge. However, considering all four groups described, the rising incidence of adolescent and young adult mental illnesses and the increasing prevalence of older adults with type 1 diabetes and insulin/food insecurity, brittle diabetes promises to increase in the future. Fig. 4 provides an algorithmic approach to brittle diabetes.
Fig. 4.
Overall approach to the treatment of brittle diabetes divided into the four groups: A. DKA and hypoglycemia due to psychological disease; B. severe glucose variability due to medical condition; C. geriatric type 1 diabetes; D. insulin and food insecurity. CGM = continuous glucose monitoring.
It is difficult to quantitate the degree of glucose instability between the four groups or between individuals. One's degree of glucose variability, no matter the etiology, is to a large degree dependent on endogenous insulin secretion. If someone with type 1 diabetes and no c-peptide secretion misses insulin injections frequently, glucose levels will surge to high levels and ketosis could potentially develop. Alternatively, someone with type 2 diabetes and significant endogenous insulin production under these same circumstances would not have such dramatic consequences. Similarly, someone with severe insulin resistance requiring hundreds of units of insulin daily may not have the glycemic variability as seen in the more common type 1 diabetes, but under the right circumstance would be more prone to hospitalization for a hyperglycemic emergency.
Perhaps the term “brittle diabetes” will eventually be of historical interest only and be replaced by more accurate descriptions as “unstable” or “dangerous” diabetes or better yet by more accurate and specific etiologies. But no diagnosis, no matter what the etiology, should be associated with a disdainful connotation. Patients with “brittle diabetes” are not failing, they are suffering, and they are at great risk. It may be our medical care that is failing to help, to recognize, and to effectively address those unique scenarios of diabetes that are complex but potentially very treatable. We must utilize both old and new technologies as well as a wholistic approach that identifies the complex psychosocial environment of the individual with diabetes over a lifetime. An exhaustive search for the causes of this presentation of diabetes is imperative as it affects people with diabetes at any point along the lifespan and is often preventable, curable, or both.
While the nomenclature changes, we now have enough understanding about the various etiologies of unstable diabetes to appropriately identify, diagnose and treat these patients more successfully than previously. Some of our treatments will be mostly medical, while others must be psychiatric and social and, in some cases, political and financial (insulin access and food insecurity) solutions must be sought. The hope is that “brittle diabetes” should someday disappear.
Note
Dr. Hirsch: conceptualization, writing-original draft
Dr. Gaudiani: conceptualization, writing-reviewing and editing
Footnotes
Declaration of competing interest
Dr. Hirsch receives research grants to his institution from Medtronic Diabetes and Insulet. He consults for Abbott Diabetes Care, Bigfoot, and Roche. Dr. Gaudiani has no conflicts of interest.
References
- 1.Woodyatt RT: Diabetes mellitus. In A Textbook of Medicine, 3rd edition. Cecil R. L., Ed Philadelphia, W.B. Saunders Company, 1934: 628.
- 2.Tattersall R.B. Brittle diabetes revisited: the third Arnold bloom memorial lecture. Diabet Med. 1997:99–110. doi: 10.1002/(SICI)1096-9136(199702)14:2<99::AID-DIA320>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Schade D.S., Drumm D.A., Duckworth W.C., Eaton R.P. The etiology of incapacitating, brittle diabetes. Diabetes Care. 1985;8:12–20. doi: 10.2337/diacare.8.1.12. [DOI] [PubMed] [Google Scholar]
- 4.Johnson-Rabbitt B., Seaquist E.R. Hypoglycemia in diabetes: the dark side of diabetes treatment. A patient-centered review. J Diabetes. 2019;11:711–718. doi: 10.1111/1753-0407.12933. [DOI] [PubMed] [Google Scholar]
- 5.Foster NC, Beck RW, Miller KM, et al: State of T1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diab Technol Therap 2019;21:66–72. [DOI] [PMC free article] [PubMed]
- 6.Voulgari C., Pagoni S., Paximadas S., Vinik A.I. “Brittleness” in diabetes: easier spoken than broken. Diab Technol Therap. 2012;14:835–848. doi: 10.1089/dia.2012.0058. [DOI] [PubMed] [Google Scholar]
- 7.Amiel S.A. Studies in hypoglycaemia in children with insulin-dependent diabetes mellitus. Horm Res. 1996;45:285–290. doi: 10.1159/000184807. [DOI] [PubMed] [Google Scholar]
- 8.Benbow S.J., Walsh A., Gill G.V. Brittle diabetes in the elderly. JR Soc Med. 2001;94:578–580. doi: 10.1177/014107680109401106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toni G., Berioli M.G., Cerquiglini L., Ceccarini G., Grohmann U., Principi N. Eating disorders and disordered eating symptoms in adolescents with type 1 diabetes. Nutrients. 2017;9 doi: 10.3390/nu9080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schade D.S., Drumm D.A., Eaton R.P., Sterling W.A. Factious brittle diabetes mellitus. Am J Med. 1985;78:777–784. doi: 10.1016/0002-9343(85)90283-9. [DOI] [PubMed] [Google Scholar]
- 11.Pelizza L., Pupo S. Brittle diabetes: psychopathology and personality. J Diab Complications. 2016;30:1544–1547. doi: 10.1016/j.jdiacomp.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Tieder J.S., McLeod L., Keren R., Luan X., Localio R., Mahant S. Pediatric research in inpatient settings network: variation in resource use and readmission for diabetic ketoacidosis in children’s hospitals. Pediatrics. 2013;132:229–236. doi: 10.1542/peds.2013-0359. [DOI] [PubMed] [Google Scholar]
- 13.Rhoads G.C., Orsini L.S., Crown W., Wang S., Getahun D., Zhang Q. Contribution of hypoglycemia to medical care expenditures and short-term disability in employees with diabetes. J Occup Environ Med. 2005;47:447–452. doi: 10.1097/01.jom.0000161727.03431.3e. [DOI] [PubMed] [Google Scholar]
- 14.Willen D., Cripps R., Wilson A., Wolff K., Rothman R. Interdisciplinary team care for diabetic patients by primary care physicians, advanced practice nurses, and clinical pharmacists. Clin Diabetes. 2011;29:60–68. [Google Scholar]
- 15.Moran G, Fonagy P, Kurtz A, Bolton A, Brook C: A controlled study of psychoanalytic treatment of brittle diabetes. J Am Acad Child Adolesc Psychiatry 1991;30-926-935. [DOI] [PubMed]
- 16.Lustman P.J., Clouse R.E. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005;19:113–122. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Sola E., Morilles C., Garzon S. Gomez-Balaguer, Hernanez-Mijares A: Association between diabetic ketoacidosis and thyrotoxicosis. Acta Diabetol. 2002;39:235–237. doi: 10.1007/s005920200040. [DOI] [PubMed] [Google Scholar]
- 18.Westphal S.A. Concurrant diagnosis of acromegaly and diabetic ketoacidosis. Endocr Pract. 2000;6:450–452. [PubMed] [Google Scholar]
- 19.Acharya R., Kabadi U.M. Case of diabetic ketoacidosis as an initial presentation of Cushing’s syndrome. Endocrinol Diabetes Metab Case Rep. 2017;23:16–0123. doi: 10.1530/EDM-16-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthony L.B., Sharp S.C., May M.E. Case report: diabetic ketoacidosis in a patient with glucagonoma. Am J Med Sci. 1995 Jun;309:326–327. doi: 10.1097/00000441-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Edelman E.R., Stuenkel C.A., Rutherford J.D., Williams G.H. Diabetic ketoacidosis associated with pheochromocytoma. Cleve Clin J Med. 1992;59:423–427. doi: 10.3949/ccjm.59.4.423. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Chen J., Zuo X. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obesity and Metab e-pub April. 2020;20 doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch I.B., D’Alessio D., Eng L., Davis C., Lernmark A., Chait A. Severe insulin resistance in a patient with type 1 diabetes and stiff-man syndrome treated with insulin lispro. Diabetes Res Clin Pract. 1998;41:197–202. doi: 10.1016/s0168-8227(98)00072-2. [DOI] [PubMed] [Google Scholar]
- 24.Famulla S., Hovelmann U., Fischer A., Coester H.V., Hermanski L., Kaltheuner M. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial control. Diabetes Care. 2016;39:1486–1492. doi: 10.2337/dc16-0610. [DOI] [PubMed] [Google Scholar]
- 25.Aleppo G., Calhoun P., Foster N.C., Maahs D.M., Shah V.N., Miller K.M. T1D exchange clinic network. Reported gastroparesis in adults with type 1 diabetes (T1D) from the T1D exchange clinic registry. J Diabetes Complications. 2017;31:1669–1673. doi: 10.1016/j.jdiacomp.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willard D.L., Stevenson M., Steenkamp D. Type B insulin resistance syndrome. Curr Opin Endocrinol Diabetes Obes. 2016;23:318–323. doi: 10.1097/MED.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 27.Roberts K.J., Blanco G., Webber J., Marudanayagam R., Sutcliffe R.P., Muiesan P. How severe is diabetes after pancreatectomy? A case-matched analysis. HPB (Oxford) 2014;16:814–821. doi: 10.1111/hpb.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madison LL, Lochner A, Wulff J: Ethanol-induced hypoglycemia: II. Mechanism of suppression of hepatic gluconeogenesis. Diabetes 1967;16:252–258. [DOI] [PubMed]
- 29.Allison M.G., McCurdy M.T. Alcoholic metabolic emergencies. Emer Med Clin North Am. 2014;32:293–301. doi: 10.1016/j.emc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Writing Group for the DCCT/EDIC Research Group Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, Lachin JM: association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313:45–53. doi: 10.1001/jama.2014.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller R.G., Secrest A.M., Sharm R.K., Songer T.J., Orchard T.J. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh epidemiology of diabetes complications study cohort. Diabetes. 2012;61:2987–2992. doi: 10.2337/db11-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas N.J., Jones S.E., Weedon M.N., Shields B.M., Oram R.A., Hattersley A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6:122–129. doi: 10.1016/S2213-8587(17)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Home secretary Theresa May diagnosed with type 1 diabetes. https://www.bbc.com/news/uk-politics-23413273 accessed January 18, 2020.
- 34.La Ferla R: Jerry Lewis at 90: his mouth runneth over. https://www.nytimes.com/2016/09/11/fashion/jerry-lewis-home-max-rose-movie-aging.html?mcubz=1&_r=0 accessed January 18, 2020.
- 35.Sanon V.P., Sanon S., Kanakia R., Yu H., Araj F., Oliveros R. Hypoglycemia from a cardiologist’s perspective. Clin Cardiol. 2014;37:499–504. doi: 10.1002/clc.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeRoith D., Biessels G.J., Braithwaite S.S., Casanueva F.F., Draznin B., Halter J.B. Treatment of diabetes in older adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104:1520–1574. doi: 10.1210/jc.2019-00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pratley R, Kanapka LG, Rickels MR, et al: Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes, JAMA, [in press]. [DOI] [PMC free article] [PubMed]
- 38.Gale E.A. Lancet. 2006 Nov 11;368:1626–1628. doi: 10.1016/S0140-6736(06)69672-4. [DOI] [PubMed] [Google Scholar]
- 39.Beran D, Ewen M, Lipska K, Hirsch IB, Yudkin JS: Availability and affordability of essential medicines: implications for global diabetes treatment. Current Diabetes Reports2018;18:48–58. [DOI] [PubMed]
- 40.https://www.t1international.com/media/assets/file/T1International_Report_-_Costs_and_Rationing_of_Insulin__Diabetes_Supplies_2.pdf accessed January 19, 2020.
- 41.Jones S: Another person has died after rationing insulin. Intellegencer July 15, 2019, http://nymag.com/intelligencer/2019/07/another-person-has-died-from-rationing-insulin.html accessed January 19, 2020.
- 42.Poulsen M.N., McNab P.R., Clayton M.L., Neff R.A. A systematic review of urban agriculture and food security impacts in low-income countries. Food Policy. 2015;55:131–146. [Google Scholar]
- 43.Landry M.J., Khazaee E., Markowitzz A.K. Impact of food insecurity on glycemic control amount low-income primarily Hispanic/Latino children in Los Angeles, California: a cross-sectional study. J Hunger Environ Nutr. 2019;14:709–724. doi: 10.1080/19320248.2018.1491367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marjerrison S., Cummings E.A., Glanville T. Prevalence and associations of food insecurity in children with diabetes mellitus. J Peds. 2011;158:607–611. doi: 10.1016/j.jpeds.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Berkowitz S.A., Karter A.J., Corbie-Smith G. Food insecurity, food “deserts”, and glycemic control in patients with diabetes: a longitudinal analysis. Diabetes Care. 2018;41:1188–1195. doi: 10.2337/dc17-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitale M., Dorado L., Pais V., Sidani S., Gucciardi E. Food insecurity screening among families of children with diabetes. Diabetes Spectr. 2019;32:338–348. doi: 10.2337/ds18-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight C.K., Probst J.C., Liese A.D., Jones S.J. Household food insecurity and medication “scrimping” among healthy US adults with diabetes. Prev Med. 2016;83:41–45. doi: 10.1016/j.ypmed.2015.11.031. [DOI] [PubMed] [Google Scholar]