Figure 1. Post-EAU exFoxP3 cells fail to suppress uveitis.
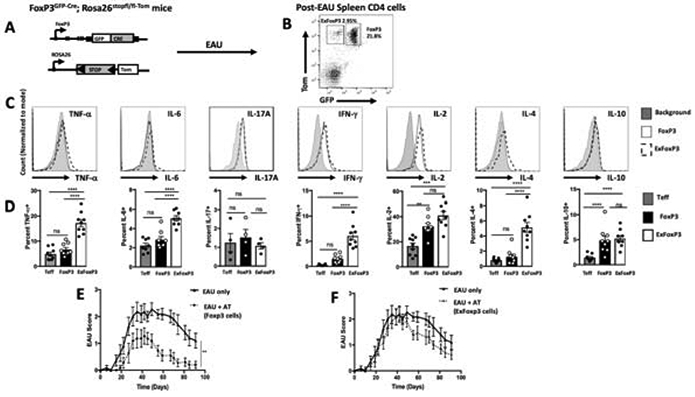
Schematic representation of the lineage tracing reporter mice, FoxP3GFP-Cre; Rosa26stopfl/fl-tdTomato (A). Post-EAU splenocytes from FoxP3GFP-Cre; Rosa26stopfl/fl-tdTomato mice were reactivated with IRBP (1-20) in vitro for 48 hours then stained with anti-CD4 antibody and analyzed by flow cytometry for CD4, GFP, and Tomato. Representative flow plot of 6 independent experiments, with 2-5 mice per experiment (n=16) is shown GFP−Tom+ (exFoxP3) cells, GFP+Tom+ (FoxP3 cells) (B). Splenocytes were activated with PMA/Ionomycin and cytokine secretion was blocked with monensin for 4-5 hrs. Cells were fixed and stained for TNF-α, IL-6, IL-17A, IFN-γ, IL-2, IL-4, and IL-10 and analyzed by flow cytometry. Representative histograms of the intracellular cytokines of cells gated on exFoxP3 (dashed line), FoxP3+ (black line), or background (light gray) are shown (C). Summary data show the mean ± SEM of frequencies with T effector cells as CD4+ (GFP−Tom−) of cytokines from 2 independent experiments with 2-5 mice per experiment (D). Post-EAU splenocytes were sorted into FoxP3 and exFoxP3 groups following reactivation with IRBPp (1-20) and transferred to recipient mice immunized for EAU. Clinical EAU scores obtained from fundus exams of EAU mice are shown. Average scores per group over time for non-recipient control mice (EAU only, n=11) are compared with scores of FoxP3-recipient mice (n=11) (E) or exFoxP3-recipient (n=13) mice (F). The graphs show the mean ± SEM scores on the indicated day per group. Results are from 3 independent experiments with 3-5 mice in each group per experiment, where each symbol on the bar graph represents one mouse. Cytokine results were analyzed with one-way ANOVA **p<0.01, ***p<0.001, ****p<0.0001, ns not significant. EAU results were analyzed with two-way ANOVA with Bonferroni post-test, **p<0.005 n.s. not significant.
