Abstract
The blackberry is a fragile fruit with a high degree of decomposition, which limits its shelf life. The effect of an edible coating (EC) based on cassava starch, whey protein, beeswax, chitosan, glycerol, stearic acid, and glacial acetic acid on the shelf life of fruit stored at 4 °C was evaluated. The physical, chemical, physical, microbiological, and sensorial quality was evaluated, comparing with a fresh control fruit. The EC had a positive effect on the physicochemical and sensorial properties (mainly in texture, flavor, and aromas), due to the reduction of physiological processes, whereas the color changes are mainly due to anthocyanin losses. After 10 days of storage, weight losses were 39.6% lower and firmness was 81.4% higher; while chitosan reduced the mold and yeast count. The EC increased the useful life of the Andean blackberries by 100%.
Keywords: Food science, Food storage, Shelf life of foods, Food quality, Material science of foods, Chemical food analysis, Rubus glaucus Benth, Antimicrobial film, Proteins, Shelf life, Storage
Food science; Food storage; Shelf life of foods; Food quality; Material science of foods; Chemical food analysis; Rubus glaucus Benth; Antimicrobial film; Proteins; Shelf life; Storage
1. Introduction
Andean blackberry (Rubus glaucus Benth) is a fruit native to tropical areas of South America, grown mainly in countries such as Colombia and Ecuador, and it is distinguished by being a non-climacteric fruit, rich in minerals (Ca, P, K, Mg, Fe) and vitamins A, C, E, K, B (Carvalho and Betancour, 2015; Ospina et al., 2019; Sinuco et al., 2013). Additionally, it is a juicy, dark red fruit with a pleasant aroma, characterized in the food industry as a fruit of versatile transformation (Arozarena et al., 2012; Kumar et al., 2017a, Kumar et al., 2017b; Dávila et al., 2017; Rojas-Llanes et al., 2014).
In recent years, the consumption of blackberries has increased, opening export markets mainly in North America and the European Union (Carvalho and Betancour, 2015), for its high nutritional value. It is an excellent natural source of antioxidant compounds such as benzoic acid, hydroxycinnamic acid, flavonoids, ellagic acid, tannins, ellagitannins, quercitin, gallic acid, anthocyanins, and cyanidins (Acosta-Montoya et al., 2010; Arozarena et al., 2012; Estupiñan et al., 2011; Kumar et al., 2017a, Kumar et al., 2017b; Mertz et al., 2007; Parada-Moreno et al., 2012; Rojas-Llanes et al., 2014; Romero and Yépez, 2015), which can contribute to the prevention of degenerative diseases (Ali et al., 2011; Carvalho and Betancour, 2015).
However, blackberry is a fruit with a high-water content (90–91%), fragile morphological structure, and undergoes continuous physicochemical and firmness changes. This makes it a highly perishable fruit susceptible to fungal contamination and large post-harvest losses under inadequate management, giving it a shelf life of 3–5 days at refrigeration temperatures (Ayala et al., 2013; Ramírez et al., 2013; Rodríguez-Barona et al., 2012; Romero and Yépez, 2015; Villegas and Albarracín, 2016). To guarantee the quality and safety of the blackberry, thermal treatments have been used; however, these directly affect the antioxidant, nutritional, and sensorial properties (Romero and Yépez, 2015; Villegas and Albarracín, 2016).
In recent years, technological alternatives have been sought to reduce high energy consumption and environmental impact, as well as to ensure the quality of fresh products (Kaushik et al., 2014; São José et al., 2014; Villegas and Albarracín, 2016). In this context, edible coatings (EC) made from composite materials help to increase the shelf life of highly perishable fruits by forming a barrier capable of retaining water vapor, breathing gases (CO2, O2, ethylene) and aromas, and mitigating the physiological processes important to fruit (Bezerra-De Aquino et al., 2015; Boesso-Oriani et al., 2014; Silva et al., 2015; Thomas et al., 2016). On the other hand, ECs are considered vehicles for transporting bioactive compounds that serve to add value to fruits and protect them microbiologically (Boesso-Oriani et al., 2014; Zheng et al., 2019).
In Andean blackberries, the application of EC has been reported with formulation from different sources: Starch (Chen et al., 2019; Pajak et al., 2019; Galindez et al., 2019), aloe vera (Ramírez et al., 2013; Ortega-Toro et al., 2017; Gutiérrez and Álvarez, 2016), sodium alginate (Salama et al., 2018; Abdel-Aziz, Salama and Sabaa, 2018; Ruan et al., 2019), gellan gum (Du et al., 2019; Wei et al., 2017; Danalache et al., 2016), gelatin (Dou et al., 2018; Jridi et al., 2019; Soo and Sarbon, 2018), and casein (Chevalier et al., 2018a, Chevalier et al., 2018b), and hydroxypropyl methylcellulose (Villegas and Albarracín, 2016), among others. These have increased the shelf life of the fruit while preserving its physical and chemical properties, as well as sensory and microbiological, with respect to the fresh blackberries. However, the application of ECs with compound matrices has not yet been reported in this fruit. In papaya, starch-based EC and commercial waxes were applied to improve firmness and texture. In mango, EC was applied based on cassava starch, pectin, and olive oil, increasing shelf life up to 12 days of storage (Estrada-Mesa et al., 2015). Strawberry was coated using an EC based on tara gum, beeswax, and shellac, reducing the physiological processes of the fruit (Pavón-Vargas and Valencia-Chamorro, 2016). On the other hand, multicomponent ECs have been formulated in order to increase the preservative properties of the EC applied in fruits, as verified in strawberries with cassava starch, isolated soy protein, and canola oil (Saavedra and Algecira, 2010) and in cape gooseberry with cassava starch, whey protein, and beeswax (López et al., 2016).
The aim of this work was to evaluate the effect of a multifunctional EC based on cassava starch (CS), whey protein (WP), beeswax (BW), chitosan (CH), glycerol (G), stearic acid (SA), and glacial acetic acid in the quality of fresh Andean blackberry during storage at 4 °C.
2. Materials and methods
2.1. Materials
Andean blackberries (Rubus glaucus Benth) from the municipality of Granada (Antioquia) with maturity degree 5 were selected with a uniform size without mechanical damage or visual fungal contamination and, then, underwent a process of washing and disinfection with 50 ppm NaClO solution (Villegas and Albarracín, 2016). This fruit without coating application was considered the control fruit. The EC was prepared from CS, WP, BW, CH, G, SA, and glacial acetic acid to dissolve the CH.
2.2. Preparation and application of the edible coating
A suspension of CS (3.5% w/w) and WP (1.16% w/w) in distilled water was initially prepared using an Ultra Turrax homogenizer (digital IKA T25) at 13000 rpm for 3 min. Then, the G suspension was added, according to the ratio of G/(CS + WP) = 2, and the system was homogenized again at 13000 rpm for 3 min. On the other hand, the total content of the CH (0.75% w/w) was dissolved in a solution of 1% v/v glacial acetic acid in distilled water at 40 °C for 2 h. Subsequently, both systems were mixed with constant stirring and heating in a water bath using a heating plate (IKA C-MAG HS 4). When 70 °C was reached, the BW (0.47% w/w) and the EA were added at a ratio of BW/EA = 5, and the heating was continued up to 85 °C. Finally, the formed emulsion was cooled to 35 °C and homogenized while cold at 21000 rpm for 1 min, then degassed in a vacuum chamber at 7.4 kPa for 45 min. Blackberry with the edible coating (B + EC) was obtained by immersing the fruit for 90 s, followed by a surface runoff for 90 s more before subsequent drying by forced convection at 30 °C for 1 h.
2.3. Characterization of the blackberry fruits
For measurement of the Andean blackberry physicochemical properties, the fruit was homogenized until a homogeneous pulp was obtained and, then, passed through qualitative filters of 90 mm at 65 g/m2. The acidity was determined by potentiometric titration with NaOH until reaching a pH of 8.2 by diluting 5g in 50 mL of distilled water (Joo et al., 2011; Villegas and Albarracín, 2016), expressing the results as malic acid (%). The total soluble solids (TSS) were determined by reading with a refractometer (Colombian Technical Standard, 1997). The pH was determined with a pH-meter (Hanna pH 211) (Villegas and Albarracín, 2016). Moisture content was determined by the modified AOAC 930.15/1990 method, where drying was carried out in a vacuum oven (Memmert VO 200) at 60 °C and 1 kPa mbar for 24 h.
Weight loss (WL) was determined by the gravimetric method (Villegas and Albarracín, 2016; Mantilla et al., 2013), according to Eq. (1), where IW is the initial weight on day 0, and FW is the final weight on control days. The WL data were reported as a percentage.
| (1) |
For determination of total phenols, antioxidant activity, and anthocyanins, the extracts were obtained by weighing 0.3 g of pulp and 9 mL of methanol: water solution (70/30 v/v), mixing for 20 min, and centrifuging at 8000 rpm for 10 min and 20 °C (Hettich Universal 320 R centrifuge). Subsequently, the supernatant was filtered with 90 mm qualitative filters (65 g/m2), shaking for 20 min, and centrifuged again under the same conditions. The phenols content was determined according to the methodology of Horvitz et al. (2017), according to the following modifications: 20 μL of the methanolic extracts and 1250 μL of 20% Na2CO3 were mixed in 480 μL of distilled water, allowed to stand for 5 min, and then, it was mixed with 250 μL of the Folin-Ciocalteu reagent diluted in distilled water in a 1/1 ratio. Afterwards, it was kept in the dark for 2 h, and the absorbance was determined at 760 nm (Thermo Fisher Evo 60 spectrophotometer), expressing the results in mg of gallic acid/100 g db.
The antioxidant activity of the extracts was determined by the ABTS and DPPH methods according to the methodology described by Mannozzi et al. (2018) with some modifications: 20 μL of methanolic extract and 2 mL of ABTS solution were mixed for 1 min, left in the dark for 7 min, and then absorbance was read at 734 nm. On the other hand, 20 μL of the extract were mixed with 1.98 mL of the DPPH solution, left at rest for 30 min, and the absorbance was read at 517 nm. The results were expressed in mg of Trolox/100 g db. The anthocyanins content was determined by the pH difference method (Kuskoski et al., 2005), preparing a buffer solution of 0.025 M at pH 1 with hydrochloric acid and potassium chloride and also pH 4.5 to 0.4 M with acetic acid and sodium acetate. 200 μL of extract was mixed with 1800 μL of the corresponding buffer solution, and the absorbance was measured against a blank at 510 and 700 nm. The final absorbance (FA) was calculated with Eq. (2) and the content of anthocyanins with Eq. (3), where MW is the molecular weight (449.2 g/mol), DF is the dilution factor, ε is the molar absorptivity (26900). The data were expressed in mg of Cyanidine 3-glucoside/100 g db.
| (2) |
| (3) |
The firmness was determined through penetration tests in the equatorial zone, according to the methodology described by Ramírez et al. (2013) with some modifications. A Stable Micro System texturometer TA.XT2i was used with accessory P/5 and with a penetration speed and distance of 2 mm/s and 15 mm, respectively. The color parameters were determined on the epicarp of the equatorial zone of the fruit, taking 4 readings at 90°. An X-Rite model SP62 spectrophotometer, D65 illuminant, 10° observer, specular component included was used, obtaining the CIE-Lab color coordinates and the color changes (ΔE) from the reflection spectra (equation 4) (Ramírez et al., 2013). ΔL, Δa, and Δb represent the differences in brightness and chromaticity a (red - green) and b (yellow - blue) respectively, between the parameter evaluated in the control time t and the time 0, both for the B + EC and for the control fruit.
| (4) |
The sensory evaluation was carried out after 0, 6, 10 days of storage, evaluating acceptance tests of color, smell/aroma, flavor, and texture, using a hedonic scale of 5 points: 1 (I dislike it a lot) and 5 (I like it very much) with 30 consumer panelists (García-Mogollón et al., 2010). On the other hand, the microbiological evaluation was performed based on the count of molds and yeasts (AOAC 17.2/2002), as well as total and fecal coliforms (AOAC 966.24/1998), expressed as colony forming units/g (CFU/g).
2.4. Storage
The B + EC and the control fruit were packed in perforated polypropylene boxes and stored at 4 °C for 0, 2, 4, 6, 8, and 10 days.
2.5. Statistical analysis
Determination of the dependent variables was performed in triplicate for each control time, and the results were analyzed from ANOVA, using the LSD method (least significant differences) of multiple comparisons with a confidence level of 95%. For the statistical analysis, the statistical package Statgraphics Centurion XVI was used.
3. Results and discussions
3.1. Water loss and firmness
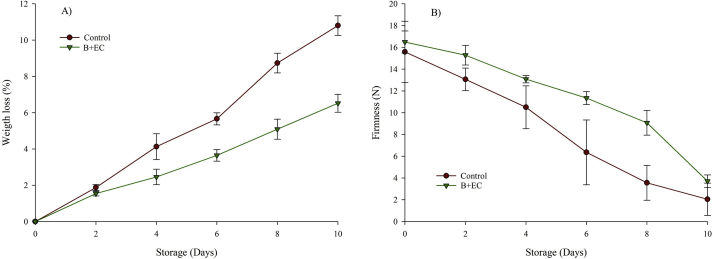
Figure 1 shows the behavior of the WF and the firmness of the B + EC and the control fruit during storage at 4 °C. The ANOVA showed significant statistical differences (p < 0.05) between the WF and the firmness with respect to the time and treatment factors. The WL showed an upward trend in both treatments, being approximately 6 and 11% at the end of storage for B + EC and fruit control, respectively, (WL in B + EC is 39.6% < control fruit), which is attributed to the mass transport and diffusion of water vapor that happens during the physiological processes of Andean blackberries (transpiration and respiration) (Ramírez et al., 2013; Villegas and Albarracín, 2016). On the other hand, the use of EC exerts a barrier to gases (O2, CO2, among others), as has been described by some authors (Ortega-Toro et al., 2014; Ayala et al., 2014), and helps reduce the respiration rate of the fruit. The incorporation of BW into the formulation increased the water vapor barrier thanks to the hydrophobic nature of this compound and the increased tortuosity it exerts on the material when the water vapor molecules pass through (Velickova et al., 2013).
Figure 1.
Weight loss (panel A) and firmness (panel B) of blackberry with the edible coating (B + EC) and blackberry without edible coating (Control) during storage at 4 °C.
Because of its plasticizing characteristics, glycerol (G) does not contribute to improving the barrier properties and increases the molecular mobility and the free volume of the material, facilitating the permeation of the molecules (Ortega-Toro et al., 2017). However, the inclusion of EA could improve the properties of barrier against water vapor due to its hydrophobic nature (Perez-Gago et al., 2005). The reduction of the WL has also been described by Villegas and Albarracín (2016) in Andean blackberries with EC based on hydroxypropyl methylcellulose and BW, as well as by Mannozzi et al. (2018) in cranberry coated with CH.
The firmness showed a descreasing trend in both treatments for all storage periods, and the surface rigidity of B + EC was greater than the control fruit (81.4% after 10 days of storage); however, the speed of change was similar. The firmness of the blackberry is linked to the respiration and transpiration processes of the fruit, occurring as enzymatic degradations cause changes in its cell wall (Horvitz et al., 2017). The application of the EC formulation acted as a barrier and reduced the physiological processes of the blackberry, and this retards the degradation of polymers present in the cell wall that adhere to peptic acids avoiding tissue softening, due to the loss of cellular turgor and the loss of extracellular and vascular air (Auras et al., 2004; Joo et al., 2011; Ramírez et al., 2013; Valle and Rodríguez, 2011). The CS-based EC provided a more elastic surface that when interacting with the fruit and resulted in reducing the loss of surface firmness. A similar situation has been reported in several dietary matrices with CS-based EC: hartón plantain (Márquez-Cardozo et al., 2015); guavas (Bezerra-De Aquino et al., 2015); peppers (Ordoñez-Bolaños et al., 2014); and blackberry (Menezes-Oliveira et al., 2013). On the other hand, the results found are also similar to those reported in blackberries covered with aloe vera mucilage (Ramírez et al., 2013), strawberries with CH-calcium-based EC (Hernández-Muñoz et al., 2008), and blueberries with CH-based EC (Mannozzi et al., 2018).
3.2. Acidity, pH, soluble solids, and total solids
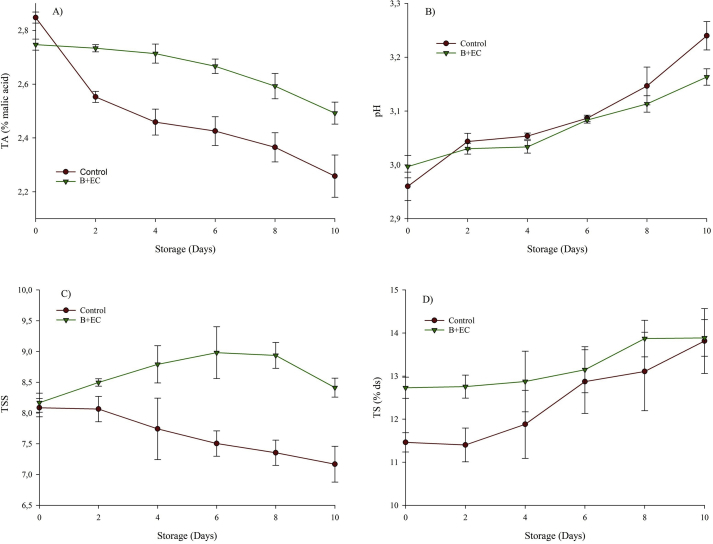
Figure 2 shows the behavior of the physicochemical properties titratable acidity (TA), pH, TSS, and total solids (TS) of B + EC and control fruit during storage at 4 °C. The maturation and the physiological processes of the blackberry affect the physicochemical properties, presenting significant statistical differences (p < 0.05) in all the parameters regarding the time and treatment factors.
Figure 2.
Titratable acidity (panel A), pH (panel B), total soluble solids (panel C), and total solids (panel D) of blackberry with the edible coating (B + EC) and blackberry without edible coating (Control) during storage at 4 °C.
During the first days of storage, the TA of the control fruit had a higher rate of change than the B + EC, varying from 2.85 to 2.26%. However, the B + EC presented an approximately constant rate of change for this parameter until day 8, observing a total decrease of 7.41%. The decrease of the TA is attributed to the oxidation of the organic acids present, which are substrates in the respiration process (Ramírez et al., 2013; Villegas and Albarracín, 2016). The behavior of the pH in both treatments was similar and consistent with the TA, increasing with the storage time. The pH was affected by the reduction in acidity. In addition, it could also be affected by the union of pectin fragments with polyphenols during the maturation process (Joo et al., 2011; Villegas and Albarracín, 2016).
The EC decreased the changes in acidity and pH, due to the effect on the physiological processes that these exert on the fruit. This behavior has been observed in several fruits with EC: blackberries with EC based on hydroxypropyl methylcellulose (Villegas and Albarracín, 2016), blackberries with EC based on mucilage from aloe vera (Ramírez et al., 2013), mango with EC to base of native and oxidized cassava starch (Figueroa and Salcedo, 2013), among others.
The TSS of the B + EC showed an increasing behavior until day 8 (8.17% → 8.94%) and an unexpected decrease to 8.4% on day 10. This increase in the TSS was consistent with the WL observed and attributable to the evaporation caused by the difference of chemical potential of the water (motive force to the transfer of mass) between the fruit and the environment (Gol et al., 2013; Ramírez et al., 2013; Velickova et al., 2013; Villegas and Albarracín, 2016); in addition, to the conversion of organic acids into sugars during the physiological processes of blackberry. However, the control fruit did not have a consistent behavior for WL, presenting a downward trend over time. This could be attributed to the consumption of sugars and organic acids in the respiration process, which is accelerated by the senescence of fruit and the weight loss due to leaching caused by the enzymatic reactions that develop in physiological processes (Gol et al., 2013; Velickova et al., 2013). This decreasing behavior was also observed by Menezes-Oliveira et al. (2013), where the blackberry control fruit had an TSS loss from 10.13 to 8.43. The opposite occurred in research presented by Ramírez et al. (2013) and Villegas and Albarracín (2016), where the blackberry control fruits increased the TSS over time.
In the case of TS, their behavior was consistent with the WL in both products, increasing over time and observing the greatest differences between the treatments in the first 4 days of storage because the driving force to the WL is greater. Some authors have attributed the increase of TS to the presence of EC on the surface of the fruit (Lopes-Carvalho et al., 2016; Mannozzi et al., 2018).
3.3. Total phenols, anthocyanins, and antioxidant activity
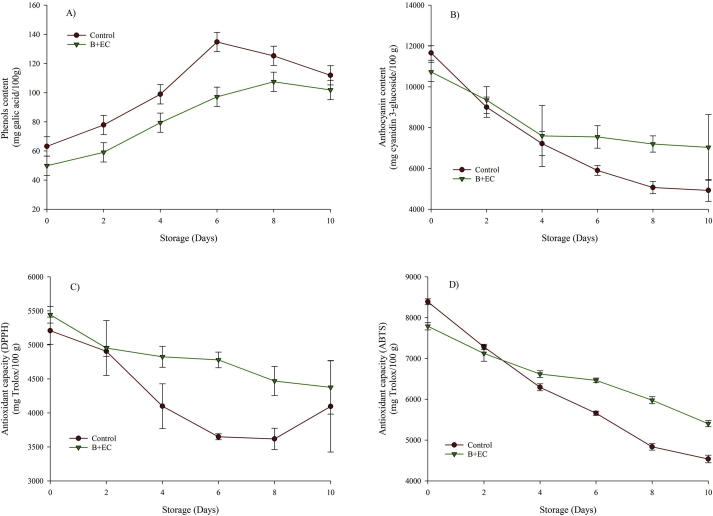
Figure 3 shows the behavior of the total phenols, anthocyanins, and the DPPH and ABTS antioxidant activity of the B + EC and the control fruit during storage at 4 °C. All these parameters presented significant statistical differences (p < 0.05) regarding the time and treatment factors.
Figure 3.
Phenols content (panel A), anthocyanin content (panel B), DPPH antioxidant capacity (panel C) and ABTS antioxidant capacity (panel D) of blackberry with the edible coating (B + EC) and blackberry without edible coating (Control) during storage at 4 °C.
The total phenols content presented a similar behavior in both treatments, with an increasing trend, but it was always higher in the control fruit than in the B + EC. The control fruit reached a maximum value at 6 days (13475.2 ± 656.7 mg gallic acid/100 g db) and subsequently decreased to 11191.2 ± 825.0 mg gallic acid/100 g db. Also, the B + EC reached its maximum value at 8 days and then was steady until day 10 (10188.8 ± 663.6 mg gallic acid/100 g db). This increase in phenolic content is attributed to the possible synthesis of compounds with antioxidant capacity during the physiological processes of the fruit, which has been also observed in plums with EC + CH (Kumar et al., 2017a, Kumar et al., 2017b). However, some authors have observed phenolic compounds obtained by Folin-Ciocalteu and are assumed to be compounds such as ascorbic acid, reducing sugars, soluble proteins among others (Oms-Oliu et al., 2008), which can explain their increase during storage. The reduction of phenolic compounds over the last days of storage can be attributed to the decomposition of the cellular structure during senescence (Gol et al., 2013).
The content of monomeric anthocyanins in both treatments presented a similar behavior and a faster change during the first 4 days. Subsequently, the B + EC showed asymptotic behavior until the 10th day, reaching 73.4 ± 10.1 mg Cyanidin 3-glucoside/100 g db and a total degradation of 31.6%. The control fruit reached the asymptotic behavior on day 8 reaching 49.9 mg Cyanidin 3-glucoside/100 g db with a total degradation of 57.2%. The decrease of anthocyanins in the blackberry was attributed to the instability of the pigment during processing and storage, generating colorless and insoluble derivatives (Ayala et al., 2012; Da Silva et al., 2014). The results obtained had a behavior similar to that reported in strawberries with EC based on CS and propolis extract (Thomas et al., 2016). On the other hand, strawberry with EC based on CH anthocyanins presented the opposite behavior and increased over time, attributed to the synthesis of anthocyanins during the physiological processes of the fruit (Gol et al., 2013).
For the control fruit and the B + EC, the mean DPPH values fluctuated between (5209.5–4095.6) and (5443.7–4376.7) mg of Trolox per 100 g db, while the ABTS was between (8389.6–4536.9) and (7790.9–5402.5) mg of Trolox per 100 g db respectively. This demonstrated that the ABTS technique is more sensitive to the determination of phenolic compounds in aqueous media, as reported by Kuskoski et al. (2005). The antioxidant capacity decreased with the increase of storage time, observing a positive effect of the EC on the protection of the Andean blackberry, which contributed to lower loss of its antioxidant activity. This can be due to the high barrier against the O2 of the EC based on CS and the decrease in the interaction with light. This decrease in antioxidant capacity is mainly associated with the loss of anthocyanins present in the fruit, which are the most important in this food matrix (Mannozzi et al., 2018; Bernal et al., 2014). However, some authors have reported an increase in antioxidant capacity due to the synthesis of secondary metabolites such as polyphenols (Ayala et al., 2014).
3.4. Color changes
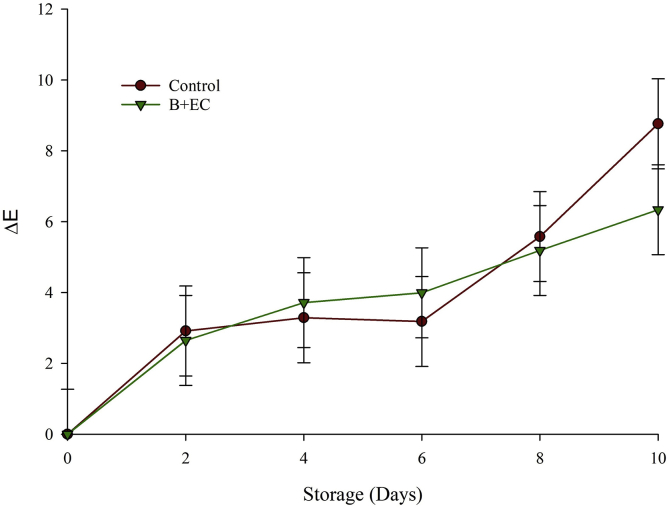
Figure 4 shows the color change (ΔE) of the B + EC and the control fruit during storage at 4 °C. The ANOVA showed significant differences (p < 0.05) in the ΔE with respect to time, whereas there were no significant differences (p > 0.05) with respect to the treatment factor. Both the B + EC and control fruit showed a tendency to increase ΔE over time, a behavior that has also been reported in aloe vera-coated blackberries (Ramírez et al., 2013).
Figure 4.
Color changes (ΔE) of blackberry with the edible coating (B + EC) and blackberry without edible coating (Control) during storage at 4 °C.
This situation could be attributed to various physicochemical and physical phenomena that both systems experience during storage: A) Anthocyanins that are structurally polyphenolic can be oxidized by the action of polyphenoloxides, B) co-pigmentation process that promotes the formation of polymers from condensation reactions of anthocyanins and other phenolic compounds, C) formation of adducts or complexes between anthocyanins and quinones generated during the oxidation of polyphenols and D) weight loss (WL) that occur during storage, caused by loss of water, especially on the fruits' surface (Mannozzi et al., 2018; Kumar et al., 2017a, Kumar et al., 2017b; Ramírez et al., 2013; Fang et al., 2007).
3.5. Microbiological analysis
Table 1 presents the counts for total coliforms, fecal coliforms, yeast, and mold of the B + EC and the control fruit during storage at 4 °C. The results obtained for allow inferring that the B + EC has a greater antimicrobial capacity than the control fruit, which is mainly due to the bactericidal characteristics of the CH present in the EC (Gol et al., 2013; Kumar et al., 2017a, Kumar et al., 2017b; Simonaitiene et al., 2015). Some researchers have reported that there is CH control over the growth of pathogenic fungi, as well as coliforms and aerobic microorganisms during the storage of blackberries (Campos et al., 2011). This control is possibly attributed to the fact that this biopolymer produces chitinase, which adheres to the cell walls of microorganisms causing severe cell damage in fungi and interfering with the secretion of enzymes that increase the senescence of the fruit (Gol et al., 2013; Hernández-Muñoz et al., 2008; Velickova et al., 2013). Some authors reported similar behaviors in strawberries coated with CH and lemon essential oil (Perdones et al., 2012), strawberries with CH (Wang and Gao, 2013), blueberries coated with CH and procyanidins (Mannozzi et al., 2018), and blueberries treated with SemperfreshTM, CH, calcium caseinate, and sodium alginate (Duan et al., 2011).
Table 1.
Microbiological counts for B + EC and control fruit during storage at 4 °C.
| Time | Total coliforms |
Fecal coliforms |
Yeast |
Mold |
||||
|---|---|---|---|---|---|---|---|---|
| Control | B + EC | Control | B + EC | Control | B + EC | Control | B + EC | |
| 0 | 20–110 | <10 | <10 | <10 | 200–250 | <10-280 | 70–140 | <10-20 |
| 2 | 20–30 | 20 | <10 | <10 | 180–200 | 140–300 | 30–50 | <10-190 |
| 4 | <10 | <10 | <10 | <10 | <10-300 | 190–280 | <10-90 | <10-60 |
| 6 | <10-100 | 10–20 | <10 | <10 | 300–380 | 130–230 | <10-200 | 60–120 |
| 8 | <10-110 | <10 | <10 | <10 | 190–250 | <10 | 80–1200 | 20–130 |
| 10 | 20–2100 | 40–70 | <10 | <10 | 2000–4000 | 180–280 | 140–3000 | 160–430 |
Data expressed in CFU/g.
On the other hand, it was observed that the control fruit values for the total coliforms and the count of molds and yeasts do not comply with the allowable requirements in Colombian regulations (150 and 3000 CFU/g respectively), whereas fecal coliforms kept the same values throughout storage. Similar results have been reported by some investigators in Andean blackberries stored at different temperatures (8 and 18 °C) (Horvitz et al., 2017). It was noted that the counts of molds and yeasts in the B + EC over the 10 days of storage maintained at values lower than 430 and 300 CFU/g, which could be due to the first instance of EC application to the fruit, which generates a barrier capable of inhibiting the growth of fungi (Velickova et al., 2013). In strawberries coated with carboxymethylcellulose-CH, hydroxypropyl methylcellulose-CH (Gol et al., 2013) and with CH-BW (Velickova et al., 2013) a reduction in the fungal growth was observed concerning the control fruit.
3.6. Sensorial analysis
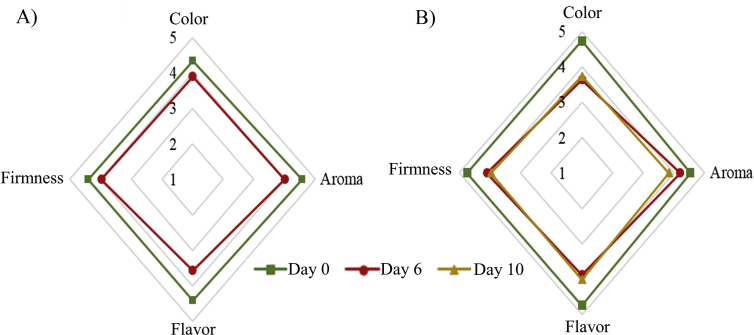
Figure 5 presents the sensory profile of the control fruit and B + EC after 0, 6, and 10 days of storage at 4 °C. In both products, it was observed that the assessment of the attributes of sensory quality such as color, aroma, taste, and texture decreased as storage days increased. It is noted that the control fruit was not evaluated on day 10, due to the presence of visual fungal contamination. However, the B + EC trends to maintain its sensorial values after 6 and 10 days, being the mean values and their standard deviation for color, aroma, flavor, and texture of 3.65 ± 1.05, 4.02 ± 0.91, 3.97 ± 1.06, 4.03 ± 0.97 respectively.
Figure 5.
Sensory profile of blackberry without edible coating (panel A) and blackberry with the edible coating (panel B) during storage at 4 °C.
In blackberries covered with aloe vera gel and carnauba wax, better sensory properties were observed with respect to the control fruits during the storage period. This is because the applied ECs delayed the physiological processes of the fruit and reduced the loss of quality by microbiological effects (Ramírez et al., 2013).
4. Conclusions
The application of the EC to Andean blackberries had a positive effect on the physicochemical properties such as pH, acidity, soluble solids, total solids, antioxidant capacity, phenolic compounds, and anthocyanins, due to the decrease of the physiological processes of the fruit such as perspiration and respiration. The color changes found are mainly due to the loss of anthocyanins that the fruit experiences during storage. The applied EC slowed the microbial growth of the fruit and conserved the sensory quality, mainly for texture, flavor, and aroma retention during 10 days of storage at 4 °C. The EC based on CS, WP, BW, CH, G, SA, and glacial acetic acid applied to the Andean blackberry is an effective alternative for its conservation, increasing its useful life up to 10 days at 4 °C, corresponding to an increase of approximately 100% when compared to what was found in the control fruit and with that reported with other researchers.
Declarations
Author contribution statement
Misael Cortés Rodríguez: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Camilo Villegas Yépez: Performed the experiments; Wrote the paper.
Jesús Humberto Gil G: Performed the experiments; Analyzed and interpreted the data.
Rodrigo Ortega Toro: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by COLCIENCIAS (Project 52774, Contract RC 33-2016) and the CEIBA Foundation (cc1085287640).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank COLCIENCIAS for the financing of the research project (52774, contract RC 33-2016), as well as the CEIBA Foundation for the funding of the master studies of one of the authors (contract cc1085287640). On the other hand, we are grateful to Michael James Stablein (University of Illinois, Urbana-Champaign) for the critical review of the manuscript.
References
- Abdel Aziz M.S., Salama H.E., Sabaa M.W. Biobased alginate/castor oil edible films for active food packaging. LWT Food Sci. Technol. 2018;96:455–460. [Google Scholar]
- Acosta-Montoya Ó., Vaillant F., Cozzano S., Mertz C., Pérez A.M., Castro M.V. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. 2010;119(4):1497–1501. [Google Scholar]
- Ali L., Svensson B., Alsanius B.W., Olsson M.E. Late season harvest and storage of Rubus berries-Major antioxidant and sugar levels. Sci. Hortic. 2011;129(3):376–381. [Google Scholar]
- Arozarena I., Ortiz J., Hermosín-Gutiérrez I., Urretavizcaya I., Salvatierra S., Córdova I., Marín-Arroyom M.R., Noriega M.J., Navarro M. Color, ellagitannins, anthocyanins, and antioxidant activity of Andean blackberry (Rubus glaucus benth.) wines. J. Agric. Food Chem. 2012;60:7463–7473. doi: 10.1021/jf300924z. [DOI] [PubMed] [Google Scholar]
- Auras R., Harte B., Selke S. Effect of water on the oxygen barrier properties of poly(ethylene terephthalate) and polylactide films. J. Appl. Polym. Sci. 2004;92(3):1790–1803. [Google Scholar]
- Ayala L.C., Valenzuela C.P., Bohorquez Y. Efecto de un recubrimiento comestible a base de alginato de sodio y iones de calcio sobre la calidad de mora castilla (Rubus glaucus Benth) Vitae. 2012;19:S129–S131. http://www.redalyc.org/articulo.oa?id=169823914035 [Google Scholar]
- Ayala L.C., Valenzuela C.P., Bohorquez Y. Effect of an edible crosslinked coating and two types of packaging on antioxidant capacity of castilla blackberries. Food Sci. Technol. 2014;34(2):281–286. [Google Scholar]
- Ayala L.C., Valenzuela C.P., Bohórquez Y. Caracterización fisicoquímica de mora de Castilla (Rubus glaucus Benth) en seis estados de madurez. Biotecnología En El Sector Agropecuario y Agroindustrial. 2013;11(2):10–18. [Google Scholar]
- Bernal L., Melo L., Díaz-Moreno C. Evaluation of the antioxidant properties and aromatic profile during maturation of the blackberry (Rubus glaucus benth) and the Bilberry (Vaccinium meridionale Swartz) Rev. Fac. Nac. Agron. 2014;67(45):7209–7218. [Google Scholar]
- Bezerra-De Aquino A., Fitzgerald-Blank A., De Aquino-Santana L. Impact of edible chitosan-cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 2015;171:108–116. doi: 10.1016/j.foodchem.2014.08.077. [DOI] [PubMed] [Google Scholar]
- Boesso-Oriani V., Molina G., Chiumarelli M., Pastore C., Dupas-Hubinger M. Properties of cassava starch-based edible coating containing essential oils. J. Food Sci. 2014;79(2):E189–E194. doi: 10.1111/1750-3841.12332. [DOI] [PubMed] [Google Scholar]
- Campos R., Kwiatkowski A., Clemente E. Post-harvest conservation of organic strawberries coated with cassava starch and chitosan. Rev. Ceres. 2011;58(5):554–560. http://www.redalyc.org/articulo.oa?id=305226809004 [Google Scholar]
- Carvalho C.P., Betancour J.A. Quality characterization of Andean blackberry fruits (Rubus glaucus Benth.) in different maturity stages in Antioquia. Agron. Colomb. 2015;33(1):74–83. [Google Scholar]
- Chen Y., Yu L., Ge X., Liu H., Ali A., Wang Y., Chen L. Preparation and characterization of edible starch film reinforced by laver. Int. J. Biol. Macromol. 2019;129:944–951. doi: 10.1016/j.ijbiomac.2019.02.045. [DOI] [PubMed] [Google Scholar]
- Chevalier E., Assezat G., Prochazka F., Oulahal N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocolloids. 2018;75:182–191. [Google Scholar]
- Chevalier E., Chaabani A., Assezat G., Prochazka F., Oulahal N. Casein/wax blend extrusion for production of edible films as carriers of potassium sorbate—a comparative study of waxes and potassium sorbate effect. Food Packag. Shelf. 2018;16:41–50. [Google Scholar]
- Danalache F., Carvalho C.Y., Alves V.D., Moldão-Martins M., Mata P. Optimisation of gellan gum edible coating for ready-to-eat mango (Mangifera indica L.) bars. Int. J. Biol. Macromol. 2016;84:43–53. doi: 10.1016/j.ijbiomac.2015.11.079. [DOI] [PubMed] [Google Scholar]
- Da Silva M., Dos Santos F., Freire M., Correa L. Avaliação dos efeitos da radiação gama na conservação da qualidade da polpa de amora preta (Rubus spp. L.) Rev. Bras. Frutic. 2014;36(3):620–627. http://dx.xoi.org/10.1590/0100-2945-218/13 [Google Scholar]
- Dávila J.A., Rosenberg M., Cardona C.A. A biorefinery for efficient processing and utilization of spent pulp of Colombian Andes Berry (Rubus glaucus Benth.): experimental, techno-economic and environmental assessment. Bioresour. Technol. 2017;223:227–236. doi: 10.1016/j.biortech.2016.10.050. [DOI] [PubMed] [Google Scholar]
- Dou L., Li B., Zhang K., Chu X., Hou H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018;118:1377–1383. doi: 10.1016/j.ijbiomac.2018.06.121. [DOI] [PubMed] [Google Scholar]
- Du Y., Sun J., Wang L., Wu C., Gong J., Lin L., Mu R., Pang J. Development of antimicrobial packaging materials by incorporation of gallic acid into Ca2+ crosslinking konjac glucomannan/gellan gum films. Int. J. Biol. Macromol. 2019;137:1076–1085. doi: 10.1016/j.ijbiomac.2019.06.079. [DOI] [PubMed] [Google Scholar]
- Duan J., Wu R., Strik B.C., Zhao Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011;59(1):71–79. [Google Scholar]
- Estrada-Mesa E.M., Padilla-Reyes F., Marquez-Cardozo C.J. Efecto de recubrimientos protectores sobre la calidad del mango (Mangifera indica L.) en poscosecha. Rev U.D.C.A. 2015;18(1):181–188. [Google Scholar]
- Estupiñan D.C., Schwartz S.J., Garzón G.A. Antioxidant activity, total phenolics content, anthocyanin, and color stability of isotonic model beverages colored with Andes berry (Rubus glaucus Benth) anthocyanin powder. J. Food Sci. 2011;76(1):S26–34. doi: 10.1111/j.1750-3841.2010.01935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Zhang M., Sun Y., Sun J. Polyphenol oxidase from bayberry (Myrica rubra Sieb. et Zucc.) and its role in anthocyanin degradation. Food Chem. 2007;103(2):268–273. [Google Scholar]
- Figueroa J.A., Salcedo J.G. Effect of edible coatings based on native and oxidized cassava starch on quality of mango (Tommy Atkins) Temas Agrarios. 2013;18(2):94–105. [Google Scholar]
- Galindez A., Daza L.D., Homez-Jara A., Eim V.S., Váquiro H.A. Characterization of ulluco starch and its potential for use in edible films prepared at low drying temperature. Carbohydr. Polym. 2019;215:143–150. doi: 10.1016/j.carbpol.2019.03.074. [DOI] [PubMed] [Google Scholar]
- García-Mogollón C., Cury-Regno K., Dussán-Sarria S. Evaluación poscosecha y estimación de vida útil de guayaba fresca utilizando el modelo de Weibull. Acta Agronómica. 2010;59(3):347–355. [Google Scholar]
- Gol N.B., Patel P.R., Ramana-Rao T.V. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013;85:185–195. [Google Scholar]
- Gutiérrez T.J., Álvarez K. Physico-chemical properties and in vitro digestibility of edible films made from plantain flour with added Aloe vera gel. J. Funct. Foods. 2016;26:750–762. [Google Scholar]
- Hernández-Muñoz P., Almenar E., Valle V., Velez D., Gavara R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria×ananassa) quality during refrigerated storage. Food Chem. 2008;110(2):428–435. doi: 10.1016/j.foodchem.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Horvitz S., Chanaguano D., Arozarena I. Andean blackberries (Rubus glaucus Benth) quality as affected by harvest maturity and storage conditions. Sci. Hortic. 2017;226:293–301. [Google Scholar]
- Joo M., Lewandowski N., Auras R., Harte J., Almenar E. Comparative shelf life study of blackberry fruit in bio-based and petroleum-based containers under retail storage conditions. Food Chem. 2011;126(4):1734–1740. doi: 10.1016/j.foodchem.2010.12.071. [DOI] [PubMed] [Google Scholar]
- Jridi M., Boughriba S., Abdelhedi O., Nciri H., Nasri R., Kchaou H., Kaya M., Sebai H., Zouari N., Nasri M. Investigation of physicochemical and antioxidant properties of gelatin edible film mixed with blood orange (Citrus sinensis) peel extract. Food Packag. Shelf. 2019;21:100342. [Google Scholar]
- Kaushik N., Kaur B.P., Rao P.S., Mishra H.N. Effect of high pressure processing on color, biochemical and microbiological characteristics of mango pulp (Mangifera indica cv. Amrapali) Innovat. Food Sci. Emerg. Technol. 2014;22:40–50. [Google Scholar]
- Kumar B., Smita K., Cumbal L., Debut A., Angulo Y. Biofabrication of copper oxide nanoparticles using Andean blackberry (Rubus glaucus Benth.) fruit and leaf. J. Saudi Chem. Soc. 2017;21:S475–S480. [Google Scholar]
- Kumar P., Sethi S., Sharma R., Manish S., Varghese E. Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci. Hortic. 2017;226:104–109. [Google Scholar]
- Kuskoski E.M., Asuero A.G., Troncoso A.M., Mancini-filho J., Fett R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência e Tecnologia de Alimentos. 2005;25(4):726–732. [Google Scholar]
- Lopes-Carvalho R., Freitas-Cabral M., Andrade-Germano T., Moita-DeCarvalho W., Montenegro-Brasil I., Gallao M.I., Herbster-Moura C.F., Almeida-Lopes M.M., Alcantara-Miranda M. Postharvest Biology and Technology Chitosan coating with trans -cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016;113:29–39. [Google Scholar]
- López D., Mora O., Arango O. Desarrollo de un recubrimiento comestible multicomponente para la conservación de uchuva (Physalis peruviana L.) Agron. Colomb. 2016;34:124–127. [Google Scholar]
- Mannozzi C., Tylewicz U., Chinnici F., Siroli L., Rocculi P., Rosa M.D., Romani S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018;251:18–24. doi: 10.1016/j.foodchem.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Mantilla N., Castell-Perez M.E., Gomes C., Moreira R.G. Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus) LWT Food Sci. Technol. 2013;51(1):37–43. [Google Scholar]
- Márquez-Cardozo C.J., Palacín-Beltrán J.R., Fuentes-Berrio L. Effect of cassava-starch coatings with ascorbic acidic and N-acetylcysteine on the quality of harton plantain (Musa paradisiaca) Rev. Fac. Nac. Agron. 2015;68(2):7689–7701. [Google Scholar]
- Menezes-Oliveira D., Lourenzi C., Kwiatkowski A., Clemente E. Biodegradable coatings on the postharvest of blackberry stored under refrigeration. Rev. Cienc. Agron. 2013;44(2):302–309. http://www.redalyc.org/articulo.oa?id=195325760012 [Google Scholar]
- Mertz C., Cheynier V., Günata Z., Brat P. Analysis of phenolic compounds in two blacberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid cromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007;55(21):8616–8624. doi: 10.1021/jf071475d. [DOI] [PubMed] [Google Scholar]
- Oms-Oliu G., Soliva-Fortuny R., Martín-Belloso O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008;50(1):87–94. [Google Scholar]
- Ordoñez-Bolaños D., Zuñiga-Camacho D., Hoyos-Concha J., Mosquera-Sánchez S., Mosquera-Sánchez L. Efecto de recubrimiento de almidón de yuca modificado y aceite de tomillo aplicado al pimiento (Capsicum annuum) Rev. Mexic. Cien Agric. 2014;5(5):795–805. [Google Scholar]
- Ortega-Toro R., Jiménez A., Talens P., Chiralt A. Effect of the incorporation of surfactants on the physical properties of corn starch films. Food Hydrocolloids. 2014;38:66–75. [Google Scholar]
- Ortega-Toro R., Collazo-Bigliardi S., Roselló J., Santamarina P., Chiralt A. Antifungal starch-based edible films containing Aloe vera. Food Hydrocolloids. 2017;72:1–10. [Google Scholar]
- Ospina M., Montaña-Oviedo K., Díaz-Duque Á., Toloza-Daza H., Narváez-Cuenca C.E. Utilization of fruit pomace, overripe fruit, and bush pruning residues from Andes berry (Rubus glaucus Benth) as antioxidants in an oil in water emulsion. Food Chem. 2019;281:114–123. doi: 10.1016/j.foodchem.2018.12.087. [DOI] [PubMed] [Google Scholar]
- Pająk P., Przetaczek-Rożnowska I., Juszczak L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019;138:441–449. doi: 10.1016/j.ijbiomac.2019.07.074. [DOI] [PubMed] [Google Scholar]
- Parada-Moreno J., Romero-Jiménez C., Yépez-Villarreal B. Aplicación de ultrasonido en el procesamiento de mora de Castilla (Rubus glaucus Benth): efecto sobre la calidad funcional y evaluación como pretratamiento al secado convectivo. Rev. Alimentos Hoy. 2012;21(27):15–38. [Google Scholar]
- Pavón-Vargas D., Valencia-Chamorro S. Efecto de recubrimientos comestibles compuestos a base de goma tara en alidad poscosecha de frutilla (Fragaria ananassa) Rev. Iberoamericana Tecnol Postcosecha. 2016;17(1):65–70. http://www.redalyc.org/articulo.oa?id=81346341009 [Google Scholar]
- Perdones A., Sánchez-González L., Chiralt A., Vargas M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012;70:32–41. [Google Scholar]
- Perez-Gago M.B., Serra M., Alonso M., Mateos M., del Río M. Effect of whey protein- and hydroxypropyl methylcellulose-based edible composite coatings on color change of fresh-cut apples. Postharvest Biol. Technol. 2005;36(1):77–85. [Google Scholar]
- Rojas-Llanes P.J., Martínez J., Stashenko E. Contenido de compuestos fenólicos y capacidad antioxidante de extractos de mora (Rubus glaucus Benth) obtenidos bajo diferentes condiciones. Vitae. 2014;21(3):218–227. [Google Scholar]
- Ramírez J., Aristizábal I., Restrepo J. Conservación de mora de castilla mediante la aplicación de un recubrimiento comestible de gel de mucílago de penca de sábila. Vitae. 2013;20(3):172–183. [Google Scholar]
- Rodríguez-Barona S., Zuluaga-Pava Y., Cruz-Ríos D. Producto potencialmente simbiótico a partir de mora de castilla (Rubus glaucus) aplicando impregnación a vacío. Sci. Agrop. 2012;3:273–278. [Google Scholar]
- Romero C.A., Yépez B.D. Ultrasound as pretreatment to convective drying of Andean blackberry (Rubus glaucus Benth) Ultrason. Sonochem. 2015;22:205–210. doi: 10.1016/j.ultsonch.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Ruan C., Zhang Y., Wang J., Sun Y., Gao X., Xiong G., Liang J. Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int. J. Biol. Macromol. 2019;134:1038–1044. doi: 10.1016/j.ijbiomac.2019.05.143. [DOI] [PubMed] [Google Scholar]
- Saavedra N., Algecira N. Evaluación de películas comestibles de almidón de yuca y proteína aislada de soya en la conservación de fresas. Nova. 2010;8(14):171–182. [Google Scholar]
- Salama H.E., Abdel Aziz M.S., Sabaa M.W. Novel biodegradable and antibacterial edible films based on alginate and chitosan biguanidine hydrochloride. Int. J. Biol. Macromol. 2018;116:443–450. doi: 10.1016/j.ijbiomac.2018.04.183. [DOI] [PubMed] [Google Scholar]
- São José J., Andrade N.J., Ramos A., Vanetti M.C., Stringheta P.C., Chaves J.B. Decontamination by ultrasound application in fresh fruits and vegetables. Food Contr. 2014;45:36–50. [Google Scholar]
- Silva K.S., Garcia C.C., Amado L.R., Mauro M.A. Effects of edible coatings on convective drying and characteristics of the dried pineapple. Food Bioprocess Technol. 2015;8(7):1465–1475. [Google Scholar]
- Simonaitiene D., Brink I., Sipailiene A., Leskauskaite D. The effect of chitosan and whey proteins-chitosan films on the growth of Penicillium expansum in apples. J. Sci. Food Agric. 2015;95(7):1475–1481. doi: 10.1002/jsfa.6846. [DOI] [PubMed] [Google Scholar]
- Sinuco D.C., Steinhaus M., Osorio C., Schieberle P. Quantitation of the odour-active compounds in Andes berry (Rubus glaucus Benth) fruit using the molecular sensory approach. Eur. Food Res. Technol. 2013;236(2):373–378. [Google Scholar]
- Soo P.Y., Sarbon N.M. Preparation and characterization of edible chicken skin gelatin film incorporated with rice flour. Food Packag. Shelf. 2018;15:1–8. [Google Scholar]
- Thomas A., Resende-Nassur R., Vilas-Boas A., De Olivera-Lima L. Cassava starch edible coating incorporated with propolis on bioactive compounds in strawberries. Ciencia e Agrotecnologia. 2016;40(1):87–96. [Google Scholar]
- Valle M.E., Rodríguez G. Evaluación de vitamina C por HPLC en el desarrollo postcosecha del tomate (Solanum lycopersicum v . Dominator) Rev Eciperú. 2011;8(1):48–53. [Google Scholar]
- Velickova E., Winkelhausen E., Kuzmanova S., Alves V.D., Moldão-Martins M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT Food Sci. Technol. 2013;52(2):80–92. [Google Scholar]
- Villegas C., Albarracín W. Aplicación y efecto de un recubrimiento comestible sobre la vida útil de la mora de castilla (Rubus glaucus benth) Vitae. 2016;23(3):202–209. [Google Scholar]
- Wang S.Y., Gao H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.) LWT Food Sci. Technol. 2013;52(2):71–79. [Google Scholar]
- Wei Y.C., Cheng C.H., Ho Y.C., Tsai M.L., Mi F.L. Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocolloids. 2017;69:491–502. [Google Scholar]
- Zheng K., Xiao S., Li W., Wang W., Chen H., Yang F., Qin C. Chitosan-acorn starch-eugenol edible film: physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019;135:344–352. doi: 10.1016/j.ijbiomac.2019.05.151. [DOI] [PubMed] [Google Scholar]