Graphical Abstract.
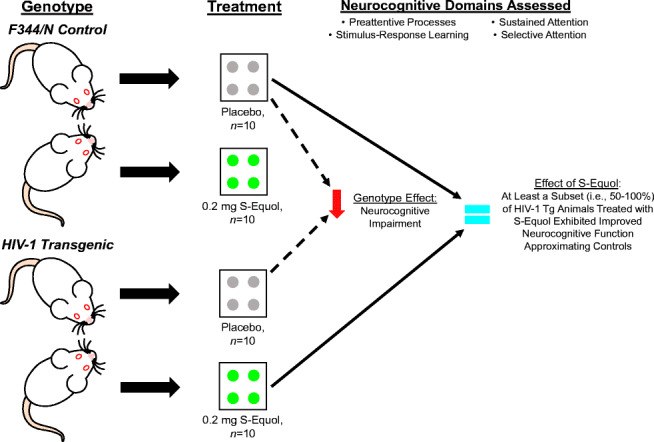
HIV-1 transgenic (Tg) and control animals were treated with either 0.2 mg S-Equol (SE) or placebo between 2 and 3 months of age (Control: Placebo, n = 10, SE, n = 10; HIV-1 Tg: Placebo, n = 10, SE, n = 10). Neurocognitive assessments, tapping preattentive processes, stimulus response learning, sustained attention and selective attention, were conducted to evaluate the utility of SE as a therapeutic for HIV-1 associated neurocognitive disorders (HAND). Planned comparisons between HIV-1 Tg and control animals treated with placebo were utilized to establish a genotype effect, revealing prominent neurocognitive impairments (NCI) in the HIV-1 Tg rat across all domains. Furthermore, to establish the utility of SE, HIV-1 Tg animals treated with SE were compared to control animals treated with placebo. Treatment with 0.2 mg SE ameliorated NCI, to levels that were indistinguishable from controls, in at least a subset (i.e., 50–100%) of HIV-1 Tg animals. Thus, SE supports an efficacious, adjunctive therapeutic for HAND.
