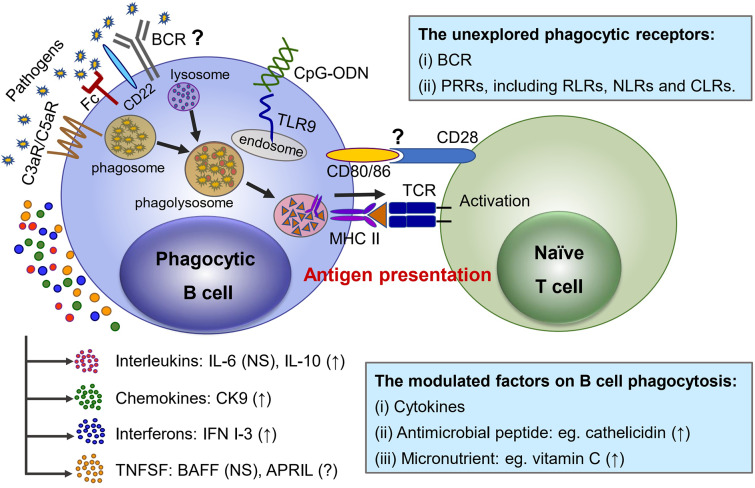
Figure 1.
Phagocytic receptors and modulating factors functioning in the phagocytosis as well as the antigen-presenting effect to naïve T cells in teleost phagocytic B cells. (1) Fc, C3a, and C5a receptors presenting at the surface of teleost B cells are involved in phagocytosis. CD22 plays regulatory roles in the micropinocytosis-dependent pathway to internalize large particles by turbot and Japanese flounder IgM+ B cells, but the contribution of BCR remains to be further studied. The PRRs recognize a wide variety of PAMPs to initiate phagocytosis, and TLR9 ligation is mediated by CpG ODN, resulting in the phagocytic capacities of splenic IgM+ B cells being up-regulated. Other PRRs, including RLRs, NLRs, and CLRs, remain to be explored. (2) Some cytokines are involved in the phagocytosis regulation of teleost B cells; specifically, IL-10, CK9, and IFN I-3 up-regulate phagocytosis, while IL-6 and BAFF have no significant effect. Other factors, such as antimicrobial peptide (cathelicidin) or micronutrients (vitamin C), have been proved to have an up-regulation function on teleost B-cell phagocytosis. (3) Pathogens (such as bacteria and viruses) are recognized by teleost B cells and engulfed into a vesicle as phagosome, with subsequent formation of maturated phagolysosome with a lysosome. The phagocytic B cells digest the internalized contents into component parts and proceed to antigen presentation with MHC II, which activates naïve T cells. However, no direct evidence has yet clarified how CD80/86 interact with CD28 in naïve T cells when B cells proceed to antigen presentation. “?” means as yet unknown; “NS” indicates no significant effect; “↑” means up-regulation.