Abbreviations
- COVID‐19
coronavirus disease 2019
- ICU
intensive care unit
- LRLT
living related liver transplant
- LT
liver transplantation
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
To the Editor:
Since the first occurrence of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic outbreak at the end of 2019,( 1 ) it was immediately clear that immune‐compromised patients, such as transplant recipients, would be at a greater risk of death and developing serious respiratory complications.( 2 ) In a similar setting, liver transplantation (LT) programs in Italy had to face a sequela of management and clinical decision‐making problems due to the extremely high incidence of SARS‐CoV‐2 in some regions of the country (Fig. 1).( 3 ) Moreover, although the Italian Transplant Authority (Centro Nazionale Trapianti) promptly released guidelines on donor management,( 4 ) the LT programs were left to pursue their own policies, even in the light of multiple logistic scenarios deriving from the different incidence rates of infection across the country (Fig. 1B). Within a similar scenario, on March 16, 2020, the Italian Society for Organ Transplantation and the Board of Liver Transplant Program Directors issued a survey to assess the initial impact of this pandemic event on the routine activity of 22 Italian LT programs, and 100% of participants completed the survey in a few days.
Fig. 1.
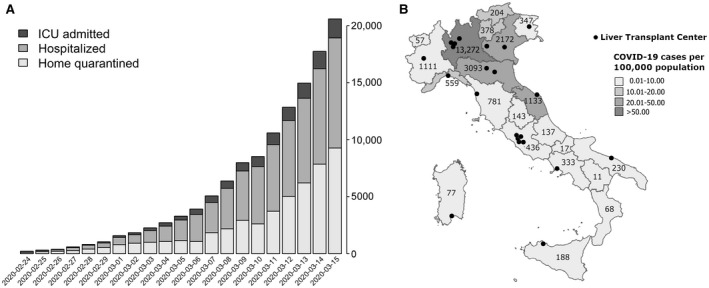
SARS‐CoV‐2 epidemic outbreak in Italy. (A) Number of confirmed COVID‐19 cases as reported by the Italian government on March 15, 2020. (B) Cartogram reporting the total number of COVID‐19 patients in each region and the number of patients per 100,000 population on March 15 as reported by Italian Government and Superior Institute of Health Care (the institutional organ of the Ministry of Health). The black dots represent all LT centers across the country. A detailed list of contributing centers is available in Supporting Table 1.
The survey included 9 questions mainly focusing on 3 aspects:
Analysis of the center’s volume of activity in February and in the first 2 weeks of March 2020 compared with the same period in 2018 and 2019.
Assessment of coronavirus disease 2019 (COVID‐19) infections in patients and health care providers in each center.
Similar evaluation in the setting of the individual living related liver transplantation (LRLT) programs.
The results of the survey are summarized in Table 1.
Table 1.
Impact of the SARS‐CoV‐2 Epidemic on the Routine Activity of 22 Italian LT Programs
| North‐Central Region | South‐Central Region | Overall | |
|---|---|---|---|
| Number of LT centers, n | 14 | 8 | 22 |
| Center policy on LT | |||
| Regular activity | 11 (79) | 6 (75) | 17 (77) |
| Reduced activity | 3 (21) | 2 (25) | 5 (23) |
| Center policy on transplant recipient follow‐up | |||
| Reduced activity | 14 (100) | 8 (100) | 22 (100) |
| Center policy on pretransplant evaluation | |||
| Regular activity | 4 (29) | 3 (38) | 7 (32) |
| Reduced activity | 9 (64) | 5 (62) | 14 (64) |
| Suspended activity | 1 (7) | 0 (0) | 1 (5) |
| Center policy on nasopharyngeal swab evaluation for LT candidates | |||
| For all potential recipients | 12 (86) | 6 (75) | 18 (82) |
| In the presence of clinical suspicion or respiratory symptoms | 2 (14) | 2 (25) | 4 (18) |
| Center volume of activity (number of LTs) from February 1 to March 15, n | |||
| 2018 | 128 | 29 | 157 |
| February 1 to February 28 | 68 | 18 | 86 |
| March 1 to March 15 | 60 | 11 | 71 |
| 2019 | 134 | 34 | 168 |
| February 1 to February 28 | 95 | 22 | 117 |
| March 1 to March 15 | 39 | 12 | 51 |
| 2020 | 121 | 35 | 156 |
| February 1 to February 29 | 98 | 21 | 119 |
| March 1 to March 15 | 23 | 14 | 37 |
| Assessment of the COVID‐19 infection in transplant recipients | |||
| Total positive for COVID‐19, n | 24 | 0 | 24 |
| Transplanted in 2020 | 5 (21) | 0 | 5 (21) |
| Required hospitalization | 17 (71) | 0 | 17 (71) |
| Required ICU admission | 3 (12) | 0 | 3 (12) |
| Dead | 5 (21) | 0 | 5 (21) |
| Assessment of the COVID‐19 infection in health care providers | |||
| Total positive to COVID‐19, n | 35 | 2 | 37 |
| Physicians | 16 (46) | 1 (50) | 17 (46) |
| Other health care providers | 19 (54) | 1 (50) | 20 (54) |
| LRLT | |||
| Total number of centers, n | 4 | 3 | 7 |
| Center policy on LT | |||
| Regular activity | 1 (25) | 2 (67) | 3 (43) |
| Reduced activity | 1 (25) | 0 | 1 (14) |
| Suspended activity | 2 (50) | 1 (33) | 3 (43) |
| Center volume of LRLT activity from February 1 to March 15, n | |||
| 2018 | 0 | 5 | 5 |
| 2019 | 0 | 2 | 2 |
| 2020 | 0 | 3 | 3 |
Data are given as n (%) unless otherwise noted.
The analysis is presented dividing all centers into 2 macroareas: north‐central Italy and south‐central Italy. The reason for this is that the 2 areas had a different incidence of the infection and that they had distinctive rates of cadaveric donation.
Overall, all centers remained open, although a reduction in the activity was noted due to the donor shortage resulting from the different patient allocation needs in Italian intensive care units (ICUs) that are now almost exclusively dedicated to the care of COVID‐19 patients.( 5 ) In the period between February 15 and March 15, all transplant programs reduced their outpatient activity both in terms of pretransplant evaluation (15 out of 22 centers, 68%) and transplant recipient follow‐up (100%). In terms of transplant activity, in the macroarea of north‐central Italy only, a reduction can be seen in the first 2 weeks of March compared with the same period in 2018 and 2019 (23 LTs versus 39 in 2018 and 60 in 2019), whereas activity in the south‐central area has not been impacted. Up to March 15, 2020, in the north‐central Italy macroarea only, we registered 24 LT recipients positive for COVID‐19 infection, of whom 3 (13%) were admitted in the ICU and 5 (21%) died. There were a total of 17 physicians among the 37 health care providers who tested positive to the infection, the majority of whom (94%) were, of course, in the northern macroarea. Also 82% of the programs performed the nasopharyngeal swab evaluation on all potential recipients upon admission for transplant, regardless of the presence of clinical suspicion or respiratory symptoms. The final section of the survey focused on LRLT: of the 7 centers performing LRLT, 3 temporarily suspended their program, whereas the remaining ones reduced their activity.
The survey paints a picture of how LT programs are managing this pandemic event in Italy and how routine activity has been impacted. Some considerations can, therefore, be made based on this preliminary analysis: the Italian system, based on a network of relationships among centers and between the centers and the Italian Transplant Authority, holds well despite the aggressiveness of this major event. Donor teams were provided by the program nearest to the donor’s hospital, regardless of where the organ was allocated, thus resulting in a limited exposure of surgeons to the epidemic widespread. Centers located in the red zone were able, with extraordinary effort, to ensure that LTs were performed for the sickest patients.
However, in the perspective of the exponentially increasing magnitude of the epidemic (Fig. 1A), other problems remain, and more time will be required to appropriately manage them. A more detailed analysis will be performed shortly.
An increasingly complex management of sick patients waiting for an organ and an elevated risk of dropout and mortality on the waiting list are the 2 major concerns of the Italian LT community. With the commitment of ICUs to primarily provide care to COVID‐19 patients, the rate of deceased donations is not expected to improve in the short term.
In addition, several patients, among those in a relatively better clinical condition (mainly oncological ones), have already been reported to have refused transplantation, due to their concern for contagion and to their deceptive perception of no urgency to be transplanted.
Priorities include solving logistical problems, such as defining safe pathways for transplant patients inside the hospitals, and identifying appropriate strategies to deliver informed consent and all information related to the potential increased risk of infection to transplant patients.
In conclusion, although we are optimistic on the overall approach of the Italian LT community to this violent outbreak, we are aware that additional critical data analysis and work are required to continue ensuring that a lifesaving procedure, such as LT, is available for many sick patients.
Supporting information
Table S1
Appendix S1
I‐BELT Study Group: Salvatore Agnes (Liver Unit, Department of Surgery, Agostino Gemelli Hospital, Catholic University, Rome, Italy), Enzo Andorno (Department of General Surgery, Istituto di Ricovero e Cura a Carattere Scientifico Azienda Ospedaliera Universitaria San Martino, Genoa, Italy), Alfonso W. Avolio (Liver Unit, Department of Surgery, Agostino Gemelli Hospital, Catholic University, Rome, Italy), Umberto Baccarani (Surgery and Transplantation, Department of Medicine, University of Udine, Italy), Amedeo Carraro (Department of Surgery, University Hospital of Verona, Italy), Matteo Cescon (Department of General Surgery and Transplantation, Bologna, Italy), Umberto Cillo (Department of Surgery, Oncology and Gastroenterology, University of Padua, Italy), Michele Colledan, (Department of Surgery, Ospedale Papa Giovanni XXIII, Bergamo, Italy), Luciano De Carlis (Chirurgia generale 2 e Trapianti, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy), Paolo De Simone (Hepatobiliary Surgery and Liver Transplantation Unit, University of Pisa Medical School Hospital, Italy), Jean De Ville De Goyet (Department for the Treatment and Study of Pediatric Abdominal Diseases and Abdominal Transplantation, IRCCS – ISMETT), Fabrizio Di Benedetto (Transplantation Unit, Department of Surgery, AOU Policlinico di Modena, Italy), Giuseppe M. Ettorre (General Surgery and Transplantation Unit, San Camillo Hospital, Rome, Italy), Enrico Gringeri (Department of Surgery, Oncology and Gastroenterology, Hepatobiliary Surgery and Liver Transplantation Unit, Padova University Hospital, Italy), Salvatore Gruttadauria (Department for the Treatment and Study of Abdominal Diseases and Abdominal Transplantation, IRCCS – ISMETT), Luigi G. Lupo (Sezione Chirurgia Generale e Trapianti di Fegato, Policlinico di Bari, Italy), Vincenzo Mazzaferro (General Surgery and Liver Transplantation Unit, University of Milan, National Cancer Institute), Enrico Regalia (General Surgery and Liver Transplantation Unit, University of Milan, National Cancer Institute), Renato Romagnoli (A.O.U. Città della Salute e della Scienza di Torino, Molinette Hospital, Department of Surgical Sciences, University of Turin, Italy), Giorgio E. Rossi (Division of General Surgery and Liver Transplantation, IRCCS Foundation, Ca' Granda Maggiore Hospital, University of Milan, Italy), Massimo Rossi (Hepato‐bilio‐pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Italy), Marco Spada (Department of Surgery, Unit of Hepato‐biliary‐pancreatic Surgery, Bambin Gesù Pediatric Hospital, Rome, Italy), Giuseppe Tisone (Department of Transplant Surgery, Polyclinic Tor Vergata Foundation, Tor Vergata University, Rome, Italy), Giovanni Vennarecci (Division of General Surgery and Liver Transplantation, San Camillo Hospital, Rome, Italy), Marco Vivarelli (Hepatobiliary and Abdominal Transplantation Surgery, Department of Experimental and Clinical Medicine, Polytechnic University of Marche, Ancona, Italy), Fausto Zamboni (Liver Transplantation Center, Azienda Ospedaliera Brotzu, Cagliari, Italy).
Italian Society of Organ Transplantation (SITO): Ugo Boggi (Division of General and Transplant Surgery, Pisa University Hospital, Italy).
We hereby certify that all the authors whose names are listed here certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent‐licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
[Correction added on August 4th 2021 after first online publication: The list of contributors, originally published as Supporting Information to the article, has moved to the Appendix.]
Potential conflict of interest: Nothing to report.
The names of the participants in the I‐BELT Study Group and in the Italian Society of Organ Transplantation are included in the Supporting Material.
[Correction added on August 2, 2021, after first online publication: ]
Contributor Information
Salvatore Gruttadauria, Email: sgruttadauria@ismett.edu.
the Italian Board of Experts in Liver Transplantation (I‐BELT) Study Group, The Italian Society of Organ Transplantation (SITO):
Salvatore Agnes, Enzo Andorno, Alfonso W. Avolio, Umberto Baccarani, Amedeo Carraro, Matteo Cescon, Umberto Cillo, Michele Colledan, Luciano De Carlis, Paolo De Simone, Jean De Ville De Goyet, Fabrizio Di Benedetto, Giuseppe M. Ettorre, Enrico Gringeri, Salvatore Gruttadauria, Luigi G. Lupo, Vincenzo Mazzaferro, Enrico Regalia, Renato Romagnoli, Giorgio E. Rossi, Massimo Rossi, Marco Spada, Giuseppe Tisone, Giovanni Vennarecci, Marco Vivarelli, Fausto Zamboni, and Ugo Boggi
References
- 1. Fauci AS, Lane HC, Redfield RR. Covid‐19—navigating the uncharted. N Engl J Med 2020;382:1268‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020;26:832‐834. [DOI] [PubMed] [Google Scholar]
- 3. Gori A, Dondossola D, Antonelli B, Davide M, Laura A, Paolo R, et al. Coronavirus disease 2019 and transplantation: a view from the inside. Am J Transplant 2020. 10.1111/ajt.15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centro Nazionale Trapianti. aggiornamento delle misure di prevenzione della trasmissione dell'infezione da nuovo Coronavirus (SARS‐CoV‐2) in Italia attraverso il trapianto di organi, tessuti e cellule. http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_229_0_file.pdf. Accessed June 4, 2020.
- 5. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020;323:1545‐1546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Appendix S1


