Figure 1.
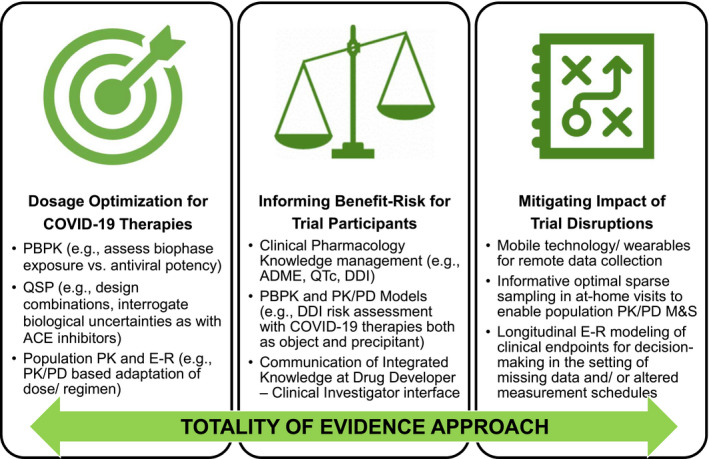
Opportunities for clinical pharmacology and model‐informed drug development during the COVID‐19 pandemic. Applications in ( left panel ) dosage optimization of COVID‐19 therapies, ( middle panel ) informing benefit/risk for clinical trial participants, and ( right panel ) mitigating impact of clinical trial disruptions, with a Totality of Evidence approach. ACE, angiotensin converting enzyme; COVID‐19, coronavirus disease 2019; DDI, drug‐drug interaction; E‐R, exposure‐response; M&S, modeling and simulation; PBPK, physiologically‐based pharmacokinetics; PD, pharmacodynamics; PK, pharmacokinetics; QSP, quantitative systems pharmacology.
