Figure 2.
CCDC61 Forms Linear Filaments via Homodimerization Mediated by the Head and Coiled-Coil Domains
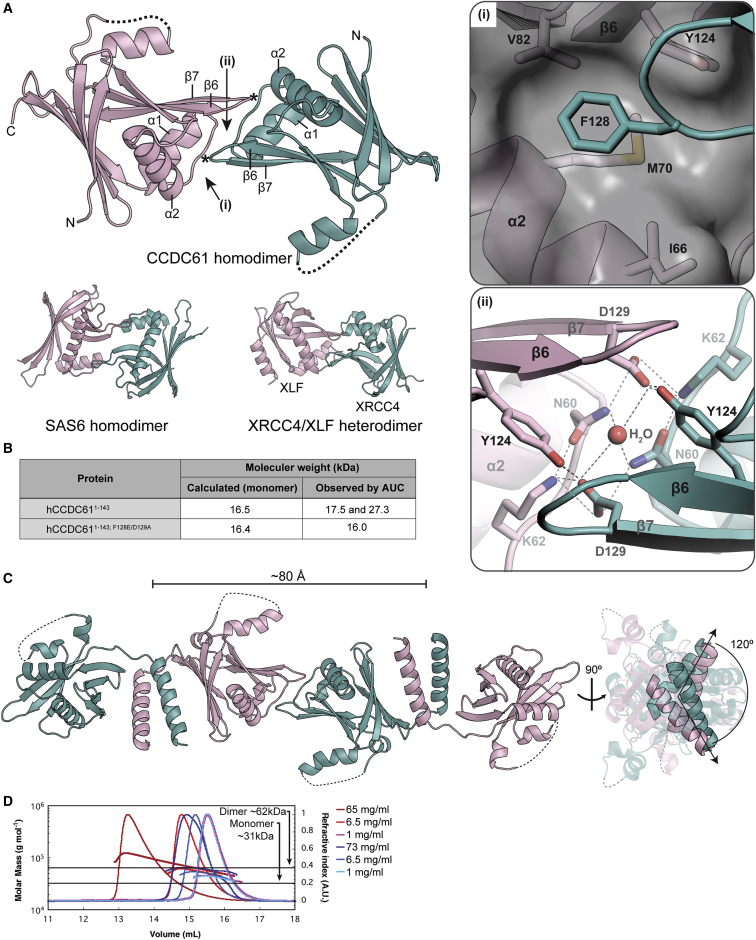
(A) Crystal structure of the head-to-head homodimer of hCCDC611−143. Missing loops are drawn with dotted lines. Key residues of the interaction interface are indicated by (i) and (ii), of which magnified views are shown in the square boxes on the right. Asterisk indicates the locations of the F128 residues. Dotted lines in panel (ii) indicate hydrogen bonds. Head-to-head dimers of SAS6 and XRCC4/XLF (PDB: 2Y3V [van Breugel et al., 2011] and 3W03 [Wu et al., 2011]) are shown at the bottom.
(B) AUC results showing that hCCDC611−143 forms homodimers in solution.
(C) Crystal structure of the zCCDC611−170 tetramer. On the right, straight arrows indicate the N-to-C direction of the coiled-coil domains. The angle between the arrows is 120°.
(D) CCDC61 forms higher-order oligomers in solution. Size-exclusion chromatography with multi-angle light scattering analysis of His6-lipoyl-zCCDC611−170 (red) and His6-lipoyl-zCCDC611−170; F129E/D130A (blue) using a Superdex S200 column at room temperature. Protein concentrations (before injection onto the column) were 1, 6.5, and 65 mg/ml (lightest to darkest red, respectively) and 1, 6.8, and 73 mg/ml (lightest to darkest blue, respectively). The minimum and maximum refractive index values of each chromatography profile were normalized to 0 and 1, respectively.
See also Figures S2 and S3.