Figure 1.
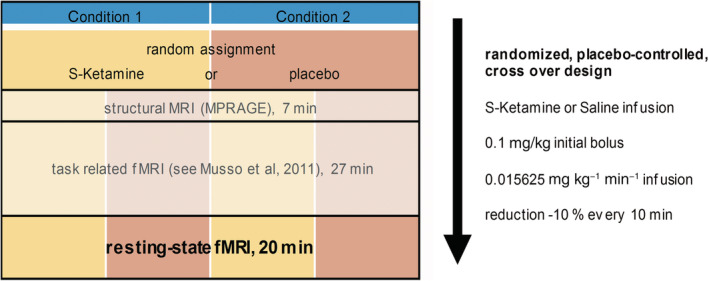
The experimental design including scanning sequences and information about drug application. During the neuroimaging investigation, subjects received in random order either a subanesthetic dose of S‐Ketamine HCl (Esketaminehydrochlorid) [Ketanest® S, Pfizer] or placebo (crossover design, 1 week apart), both administered intravenously in 0.9% NaCl. This administration was carried out as a bolus of S‐Ketamine (0.1 mg/kg during 5 min) immediately before measurements in the MRI scanner and a continuous infusion of S‐Ketamine (0.015625 mg kg−1 min−1 for the duration of the investigation, i.e., 1 hr maximum) during the measurement. To avoid a slow increase of ketamine plasma levels, a reduction by 10% every 10 min (Umbricht et al., 2000) was determined. The resting‐state fMRI measurement started 34 min after the beginning of the ketamine infusion
