Figure 2. The Endoplasmic Reticulum (ER) Proteostasis Network.
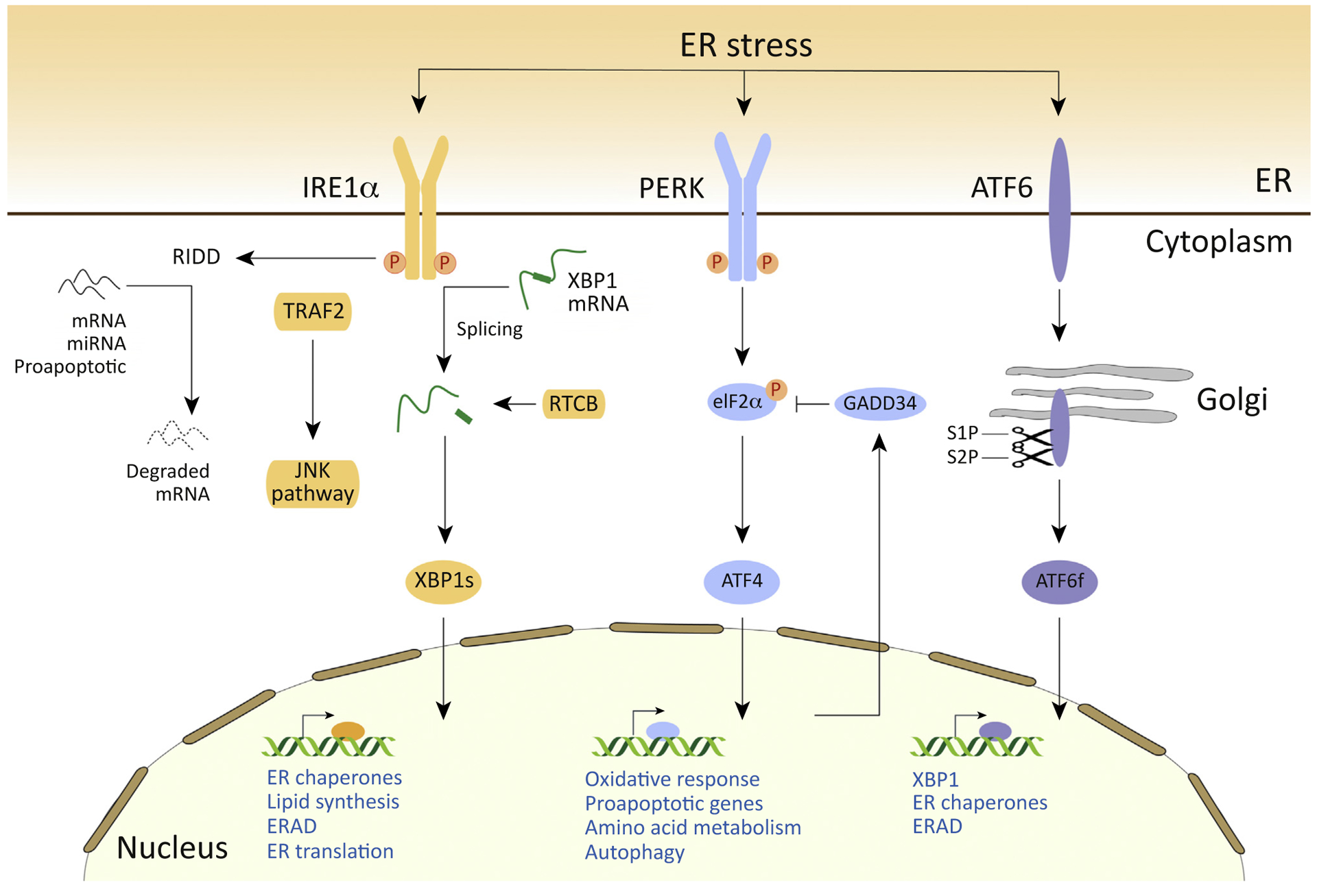
The Unfolded protein response (UPR): misfolded protein accumulation in the ER activates the UPR sensors inositol-requiring enzyme 1α (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6). Upon ER stress, ATF6 is transported to the Golgi apparatus, where it is cleaved by site 1 protease (S1P) and S2P, releasing the cytosolic ATF6 fragment (ATF6f) which operates as a transcription factor. ATF6f induces genes required for ER-associated protein degradation (ERAD) and regulates X-box binding protein 1 (XBP1) mRNA levels. ER stress also activates PERK, which phosphorylates (P) eukaryotic translation initiation factor 2α (eIF2α), which in turn inhibits global protein translation, with the exception of some mRNAs including ATF4. ATF4 induces the expression of ER chaperones, genes related to autophagy, redox control, and amino acid metabolism. ATF4 also controls genes related to apoptosis, including C/EBP-homologous protein (CHOP). Active IRE1 generates the splicing of mRNA encoding XBP1 in a reaction that is completed by the RTCB ligase, leading to the expression of an active transcription factor. Spliced XBP1 (XBP1s) upregulates ER chaperones, genes involved in the ERAD pathway, and lipid synthesis. In addition, IRE1 is associated with tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) and induces activation of c-Jun N-terminal kinase (JNK), apoptosis signal-regulating kinase (ASK1), and nuclear factor κB (NF-κB), thereby modulating autophagy and apoptosis. IRE1 endoRNase activity also induces a process known as regulated IRE1-dependent mRNA decay (RIDD) that affects different pathways, including those involved in lipid biosynthesis and apoptosis.
