Abstract
Bacteriophytochromes are photoreceptors that regulate various physiological processes induced by photoisomerization in a linear tetrapyrrole chromophore upon red/far-red light absorption. Here, we investigate the photoinduced Pfr-state isomerization mechanism of a bathy bacteriophytochrome from Pseudomonas aeruginosa combining femtosecond-resolved fluorescence and absorption methods. We observed initial coherent oscillation motions in the first one picosecond with low-frequency modes below 60 cm−1, then a bifurcation of the wavepacket with the distinct excited-state lifetimes in a few picoseconds, and finally chromophore-protein coupled ground-state conformational evolution on nanosecond time scales. Together with systematic mutational studies, we revealed the critical roles of hydrogen bonds in tuning the photoisomerization dynamics. These results provide a clear molecular picture of the Pfr-state photoisomerization, a mechanism likely applicable to the other phytochromes.
Keywords: Photoisomerization, Bifurcation, Pfr state, Fluorescence dynamics, Phytochrome
Graphical Abstract

Photosensing is essential to the survival of living organisms.1 Despite of the chemically distinct chromophores, the rhodopsin,2 xanthopsin,3 and phytochrome4 photoreceptor families all utilize a photoinduced double bond isomerization reaction in a chromophore to induce protein conformational changes. The light energy is converted to the kinetic energy in a strained chromophore, which triggers subsequent signaling to control various biological functions.5,6 The photoisomerization mechanisms of rhodopsins and xanthopsins have been intensively studied.7,8 However, the photochemical dynamics of phytochromes is less well-understood. Upon excitation, the photoinduced isomerization takes place at the C15=C16 double bond (Figure 1A) of the chromophore in phytochromes.9 Extensive studies by femtosecond (fs)-resolved transient absorption spectroscopy have been reported.9-15 Typically, the excited-state lifetimes of the red light absorbing Pr state (15Za) is reported to be tens to hundreds of picoseconds while the far-red light absorbing Pfr state (15Ea) is usually about a few picoseconds.9,10 The origins of multiphasic decays and dramatically different photoisomerization dynamics between Pr and Pfr states are still in debate.11-15
Figure 1.

(A) Local chromophore binding-site structure of PaBphP (PDB code 3NHQ). The critical residues around chromophore (cyan) D-ring and the C-ring propionate group are highlighted in purple and orange, respectively. (B) The Pfr steady-state absorption spectra of WT (red), Q188L (green), and Y250F (purple) and the emission spectrum of the Y250F mutant at longer wavelengths. The dashed line of the emission spectrum is the lognormal fitting extension. The grey dashed lines are the WT steady-state spectra of Pr state for comparison.
To resolve these important issues, we studied the Pfr state photoisomerization dynamics of bathy bacteriophytochrome from Pseudomonas aeruginosa (PaBphP).16-18 The bathy bacteriophytochrome PaBphP has a thermostable Pfr state as the dark-adapted state and conserves the typical PAS-GAF-PHY domain architecture as the photosensory module. The chromophore molecule biliverdin IXα is buried inside the GAF domain and shielded from outside environment (Figure 1A). The chromophore A-ring is covalently linked to a cysteine residue of the N-terminal extension of the PAS domain. In the Pfr state, the chromophore B- and C-ring propionate groups form extensive hydrogen-bonding interactions with the GAF domain. Both S275 and H277 are within hydrogen-bonding distance to the C-ring propionate group while both Q188 and Y250 form hydrogen bonds with the D-ring (Figure 1A). Upon isomerization, the hydrogen-bonding interactions of Q188 and Y250 to the chromophore D-ring are broken, indicating their direct involvement of the excited-state isomerization.19 Subsequently, the local structural changes originating from the D-ring extend to other rings and surrounding protein moiety.19 Here, by combining both fs-resolved fluorescence and absorption methods, we report the ultrafast Pfr state photoisomerization mechanism of bathy bacteriophytochrome PaBphP. We systematically characterized the Pfr state photoisomerization dynamics of the WT and five mutants of Q188L, Y250F, S261A, S275A, and H277A. By careful examination of the dynamics changes in each mutant, we elucidate the photoisomerization mechanism and reveal the critical roles of the protein environment in the ultrafast Pfr state reaction.
Figure 1B shows the Pfr steady-state absorption spectra of wild-type, Q188L, and Y250F as well as the fluorescence spectrum of Y250F. The WT absorption spectrum has its peak at 750 nm with a shoulder near 680 nm. The hydrogen-bonding interactions around the chromophore D-ring are important for spectral tuning in phytochromes. The hydrogen bonds with Q188 and Y250 result in different interactions with the chromophore. For Q188L, the Pfr state absorption spectrum is red-shifted by 10 nm while in Y250F the peak is blue-shifted by 3 nm compared to the WT. The S261, S275, and H277 residues have no significant influence on the Pfr state absorption spectrum (Figure S1). The Pfr state of WT is previously considered not fluorescent and the fluorescence spectrum has not been reported.20,21 Here, we obtained the Pfr state fluorescence spectrum of Y250F mutant which displays a major peak around 820 nm and extends to 1000 nm.
To characterize the excited-state dynamics, we directly measured the fs-resolved fluorescence dynamics of WT, Q188L, S261A, S275A, and H277A gated from 820 to 940 nm (Figure S2). All the transients of each protein show a double-exponential decay behavior and are the same for all emission wavelengths. The fluorescence transients of WT and mutants gated at 860 nm are shown in Figure 2A. For WT, we obtained two components of 1 ps (56%) and 4.3 ps (44%). For mutant S261A and S275A, we obtained two components of 1 ps and 3.8 ps. For mutant H277A, we obtained two components of 0.86 ps and 3.5 ps. For mutant Q188L, we can fit a double-exponential decay of 0.4 ps (95%) and 2.9 ps (5%). These results are listed in Table 1. These dynamics clearly show the excited-state behavior and no protein solvation involved on this time scale (Figure S2). The inset in Figure 2A shows a transient spectrum of WT at 1 ps delay time with an emission peak around 845 nm.
Figure 2.
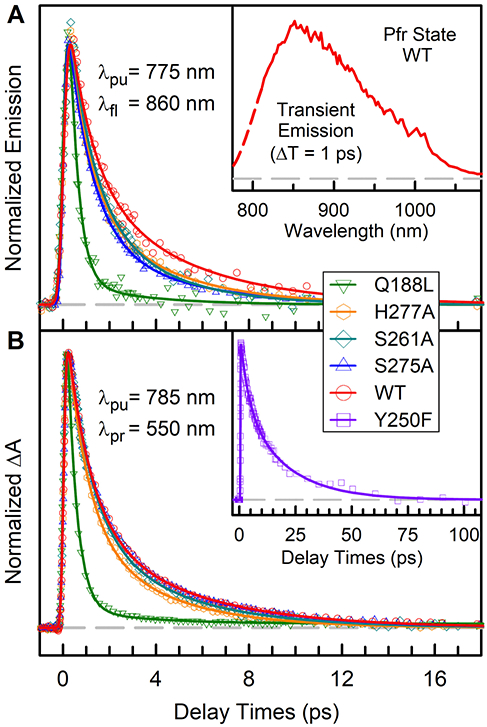
(A) Normalized femtosecond-resolved fluorescence transients of WT PaBphP and mutants gated at 860 nm. The inset shows the WT transient emission spectrum measured at time delay of 1 ps without correction of the instrumental response. (B) Normalized femtosecond-resolved absorption transients of WT PaBphP and mutants probed at 550 nm. Inset shows the Y250F transient at longer time scale. Note the ultrafast dynamics of mutant Q188L and the slow dynamics of Y250F.
Table 1:
Ultrafast dynamics in PaBphP Pfr state.a
| λfluorescence (860 nm) |
λprobe (550 nm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | S261A | S275A | H277A | Q188L | WT | S261A | S275A | H277A | Q188L | Y250F | |
| τ1 | 1.0 | 1.0 | 1.0 | 0.86 | 0.4 | 1.0 | 1.0 | 1.0 | 0.86 | 0.4 | 3.6 |
| τ2 | 4.3 | 3.8 | 3.8 | 3.5 | 2.9 | 4.3 | 3.9 | 4.3 | 3.5 | 3.0 | 19 |
| A1 | 56 | 69 | 71 | 66 | 95 | 64 | 65 | 64 | 67 | 96 | 38 |
| A2 | 44 | 31 | 29 | 34 | 5 | 36 | 35 | 36 | 33 | 4 | 62 |
Time constants are in units of ps and amplitudes are calculated relative to each other.
The Pfr state photoisomerization dynamics have never been reported by direct fluorescence measurement and only Pr state was reported before.20-23 The origin of the multiphasic decays in phytochrome photoisomerization dynamics has been proposed from protein ground-state heterogeneity.13,15 This hypothesis is mainly from the transient-absorption measurements. Mathes et al. reported that in the Pr state the absence of hydrogen bonds to the chromophore D-ring would increase the spectral heterogeneity.24 However, our results of the Q188L mutant, by removing hydrogen bond with D-ring in PaBphP, show the excited-state dynamics almost being a single-exponential decay. The observation directly contrasts with the ground-state population heterogeneity model. Instead, it suggests that the bifurcation of the wavepacket motion from the excited state is a plausible mechanism.25 Theoretical simulations of the bilin chromophore have shown the existence of competing isomerization pathways in vacuum while the local protein architecture and electrostatics selectively modulate the reaction to occur along the C15=C16 bond.26,27 In WT, the excited-state population bifurcates along the two pathways of 1 and 4.3 ps, respectively. By removing the hydrogen-bonding interaction to the D-ring in Q188L, the excited-state population is directed dominantly towards the ultrafast 0.4-ps pathway. Only with fs-resolved fluorescence detection can we determine the initial excited-state dynamics and here a bifurcation of the wavepacket motion.
To further study the Pfr excited-state dynamics and possible intermediates, we switched to the transient-absorption detection in the visible range (Figure 2B, S3-S4). Specifically, from 410 to 425 nm, we detected ground-state bleaching recovery in a few picoseconds and a negative amplitude plateau due to the photoproduct formation. From 550 to 610 nm, transient signals directly reflect the excited-state absorption with minor ground-state bleaching signal. Figure 2B shows the transients of WT and all the mutants probed at 550 nm. All the transients can be fitted with a bi-exponential decay model and are comparable to the fluorescence results (Table 1), indicating that the transient absorption signals also represent the initial excited-state dynamics. From 630 to 680 nm, the probed transient-absorption signals are a mixture of the ground-state bleaching, excited-state absorption, and ground-state product formation (Figure S3 and S4). We observed a long component in nanoseconds at several wavelengths, indicating the continuous ground-state conformational relaxation processes of the photoproduct Lumi-F intermediate.9,19 The photoproduct of Pfr state photoisomerization exhibits a distorted bond angle between the chromophore C- and D-rings. The strain is further released by the twisting of methine bridges.19 The photoproduct conformational relaxation of WT and mutants in this study occurs on the nanosecond time scales with the largest amplitude of Q188L (Table S1-S2). The Pfr state photoisomerization dynamics have also been reported in several phytochrome systems.10,12,15 Singer et al. recently reported a bi-exponential decay of 0.83 and 3.64 ps of another bathy bacteriophytochrome, in good agreement with our results.28 In general, the Pfr state photoisomerization occurs in a few picoseconds, much faster compared to the Pr state dynamics.10,28
Surprisingly, we observed coherent oscillation signals during the Pfr state photoisomerization, probed at around 630–680 nm. Figure 3 shows the dominate low-frequency mode below ~60 cm−1 with sub-picosecond dephasing times at several wavelengths from WT and mutants transients. The oscillation motion was also observed recently in Pfr state of cyanobacterial phytochrome Cph1.29 Similarly, the low-frequency coherent oscillation modes were reported in phytochromes Pr state and other photoreceptors.30-33 In photoactive yellow protein (PYP), the observed low-frequency modes of 50 and 150 cm−1 are proposed to trigger the photoisomerization.31 Also in iron-based heme proteins, the 40 cm−1 mode was observed which is originated from the core of the porphyrin.32 Furthermore, the low frequencies around 60 cm−1 also fall within the range of some protein modes. In the Pfr state, there exist extensive hydrogen-bonding interactions between the chromophore and local protein residues. The excited-state dynamics of Pfr state photoisomerization reaction may well be modulated by the chromophore-protein coupled motions. Further theoretical study is needed to elucidate the nature of the low-frequency modes in PaBphP.
Figure 3.

Normalized femtosecond-resolved ab-sorption transients and Fourier transforms of the residuals from the fit of WT PaBphP and mutants probed from 630 to 680 nm. Signals at short delay times show clear oscillations with modes below 60 cm−1.
For photoreceptors, the protein-chromophore interactions can tune the primary photochemical reaction dynamics. In PaBphP, the Q188 and Y250 are within hydrogen-bonding distance to the Pfr state chromophore D-ring. Upon photoisomerization, the hydrogen-bonding interactions will be disrupted. Bathy bacteriophytochromes usually contain Gln or Asn at the position corresponding to Q188 here.16,17 In the Q188L mutant, we observed the ultrafast dynamics (0.4 ps) by removing the hydrogen-bonding donor that is unique among bathy bacteriophytochromes. Kim et al. reported that in Cph1, the excited-state Pfr dynamics was observed mainly in 170 fs.15 The similar results between the prototypical Cph1 and PaBphP Q188L indicate the significance of protein environment in tuning the reaction dynamics. On the other hand, by mutation of Y250F, we surprisingly observed a prolonged Pfr state dynamics with two components of 3.6 and 19 ps; see Figure 2B. With both Q188 and Y250 in WT, the Pfr photoisomerization dynamics lie between those of two mutants of Q188L (faster) and Y250F (slower). In fact, Y250 seems to weaken the hydrogen-bonding interaction between Q188 and D-ring; without Y250 the dynamics become much slower and without Q188 the dynamics become much faster. Clearly, the interaction of the D-ring with Q188 is essential to control the dynamics of photoisomerization.
In the Pfr state, H277 and S275 are within hydrogen-bonding distance with the chromophore C-ring propionate group while S261 forms a hydrogen bond with the chromophore only in the Pr state (Figure 1A). The primary photoreaction starts at C15=C16 and the D-ring is shifted significantly towards the H277 upon the completion of photoisomerization in a few picoseconds. Due to further (un)twisting of the methine bridges driving an overall chromophore rotation, the C-ring propionate group breaks hydrogen bonds with S275 and H277 while forms a new hydrogen bond with S261 on the microsecond time scale. The removal of S275 should have no obvious effects on the ultrafast primary photoisomerization dynamics, as observed here. S275 is unique in bathy bacteriochrome PaBphP, providing extra stabilization through hydrogen bonding in the Pfr dark state. On the other hand, the overall Pfr photoisomerization is faster in H277A upon the deletion of the bulky side chain (Table 1). S261A shows exclusively Pfr state absorption spectra under room light condition.19 With strong red-light illumination, we can detect a mixture of Pr/Pfr state absorption in S261A, and the reversion back to Pfr state takes only a few seconds (Figure S1C). In addition, the Pfr state photoisomerization dynamics of S261A resembles the WT (Table 1). Our results suggest that the absence of Pr state in S261A is not due to the primary photoisomerization, rather than the inability of the photoproduct to undergo further ground-state conformational rearrangements, quickly back to the original Pfr state.
In summary, we reported here our systematic characterization of the Pfr state photoisomerization dynamics of bathy bacteriophytochrome PaBphP. With femtosecond-resolved fluorescence upconversion and absorption spectroscopy, we unambiguously distinguished the excited-state and ground-state dynamics. As shown in Figure 4, the excited wavepacket initially oscillates within 1 ps on the excited-state potential surface, then bifurcates into two pathways and decays in 1 and 4 ps through the conical intersection to form ground-state Lumi-F photoproduct which continues to relax on the nanosecond time scales. With mutations of key residues around the chromophore, we observed the effects of protein environment on the Pfr state dynamics. The Q188 residue is the key anchor of the Pfr state, significantly slow down the photoisomerization dynamics; the structurally conserved Y250 residue weakens such the anchor to speed up the dynamics. The mutation of H277A decrease the side chain size and accelerates the photoisomerization dynamics. Finally, the ground-state heterogeneity proposal reported in literature needs to be examined carefully. As demonstrated by this work, the bifurcated pathway model is more plausible.
Figure 4.

Schematic potential energy surfaces for PaBphP Pfr state photoisomerization, ground and excited, along the reaction coordinate. The coherent nuclear wavepacket initially oscillates and then bifurcates into the short-time (red) and long-time (purple) routes. The excited-state population decay to the ground-state through the conical intersection (CI). Photoproduct Lumi-F continues to evolve finally to the Pr state (PDB codes: 3NOP, 3NOT)
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institute of Health Grants GM118332 (to D.Z.) and EY024363 (to X.Y.). We thank Prof. Bern Kohler for the help on infrared fluorescence measurements. We also thank Drs. Meng Zhang and Xiankun Li for the initial help in experiment.
Footnotes
Supporting Information.
The following files are available free of charge.
Materials and methods, supporting figures and tables, and additional references. (PDF)
The authors declare no competing financial interests.
REFERENCES
- (1).Möglich A; Yang X; Ayers RA; Moffat K Structure and Function of Plant Photoreceptors. Annu. Rev. Plant Biol 2010, 61, 21–47. [DOI] [PubMed] [Google Scholar]
- (2).Okada T; Ernst OP; Palczewski K; Hofmann KP Activation of Rhodopsin: New Insights from Structural and Biochemical Studies. Trends Biochem. Sci 2001, 26, 318–324. [DOI] [PubMed] [Google Scholar]
- (3).Genick UK; Borgstahl GEO; Ng K; Ren Z; Pradervand C; Burke PM; Srajer V; Teng TY; Schildkamp W; McRee DE; Moffat K; Getzoff ED Structure of a Protein Photocycle Intermediate by Millisecond Time-Resolved Crystallography. Science 1997, 275, 1471–1475. [DOI] [PubMed] [Google Scholar]
- (4).Rockwell NC; Su Y; Lagarias JC Phytochrome Structure and Signaling Mechanisms. Annu. Rev. Plant Biol 2006, 57, 837–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Quail PH Phytochrome Photosensory Signalling Networks. Nat. Rev. Mol. Cell Biol 2002, 3, 85–93. [DOI] [PubMed] [Google Scholar]
- (6).Nagatani A Phytochrome: Structural Basis for Its Functions. Curr. Opin. Plant Biol 2010, 13, 565–570. [DOI] [PubMed] [Google Scholar]
- (7).Kennis JTM; Groot M Ultrafast Spectroscopy of Biological Photoreceptors. Curr. Opin. Struct. Biol 2007, 17, 623–630. [DOI] [PubMed] [Google Scholar]
- (8).Gozem S; Luk HL; Schapiro I; Olivucci M Theory and Simulation of the Ultrafast Double-Bond Isomerization of Biological Chromophores. Chem. Rev 2017, 117, 13502–13565. [DOI] [PubMed] [Google Scholar]
- (9).Dasgupta J; Frontiera RR; Taylor KC; Lagarias JC; Mathies RA Ultrafast Excited-State Isomerization in Phytochrome Revealed by Femtosecond Stimulated Raman Spectroscopy. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Schumann C; Gross R; Wolf MMN; Diller R; Michael N; Lamparter T Subpicosecond Midinfrared Spectroscopy of the Pfr Reaction of Phytochrome Agp1 from Agrobacterium tumefaciens. Biophys. J 2008, 94, 3189–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bizimana LA; Epstein J; Brazard J; Turner DB Conformational Homogeneity in the Pr Isomer of Phytochrome Cph1. J. Phys. Chem. B 2017, 121, 2622–2630. [DOI] [PubMed] [Google Scholar]
- (12).Wang C; Flanagan ML; McGillicuddy RD; Zheng H; Ginzburg AR; Yang X; Moffat K; Engel GS Bacteriophytochrome Photoisomerization Proceeds Homogeneously Despite Heterogeneity in Ground State. Biophys. J 2016, 111, 2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kim PW; Rockwell NC; Martin SS; Lagarias JC; Larsen DS Dynamic Inhomogeneity in the Photodynamics of Cyanobacterial Phytochrome Cph1. Biochemistry 2014, 53, 2818–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Nieder JB; Brecht M; Bittl R Dynamic Intracomplex Heterogeneity of Phytochrome. J. Am. Chem. Soc 2009, 131, 69–71. [DOI] [PubMed] [Google Scholar]
- (15).Kim PW; Rockwell NC; Martin SS; Lagarias JC; Larsen DS Heterogeneous Photodynamics of the Pfr State in the Cyanobacterial Phytochrome Cph1. Biochemistry 2014, 53, 4601–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yang X; Kuk J; Moffat K Crystal Structure of Pseudomonas aeruginosa Bacteriophytochrome: Photoconversion and Signal Transduction. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 14715–14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Yang X; Kuk J; Moffat K Conformational Differences Between the Pfr and Pr States in Pseudomonas aeruginosa Bacteriophytochrome. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 15639–15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Tasler R; Moises T; Frankenberg-Dinkel N Biochemical and Spectroscopic Characterization of the Bacterial Phytochrome of Pseudomonas aeruginosa. FEBS J. 2005, 272, 1927–1936. [DOI] [PubMed] [Google Scholar]
- (19).Yang X; Ren Z; Kuk J; Moffat K Temperature-Scan Cryocrystallography Reveals Reaction Intermediates in Bacteriophytochrome. Nature 2011, 479, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Holzwarth AR; Venuti E; Braslavsky SE; Schaffner K The Phototransformation Process in Phytochrome. 1. Ultrafast Fluorescence Component and Kinetic-Models for the Initial Pr→Pfr Transformation Steps in Native Phytochrome. Biochim. Biophys. Acta 1992, 1140, 59–68. [Google Scholar]
- (21).Sineshchekov VA Photobiophysics and Photobiochemistry of the Heterogeneous Phytochrome System. Biochim. Biophys. Acta, Bioenerg 1995, 1228, 125–164. [Google Scholar]
- (22).Toh KC; Stojkovic EA; van Stokkum IHM; Moffat K; Kennis JTM Proton-transfer and Hydrogen-bond Interactions Determine Fluorescence Quantum Yield and Photochemical Efficiency of Bacteriophytochrome. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 9170–9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Andel F; Hasson KC; Gai F; Anfinrud PA; Mathies RA Femtosecond Time-Resolved Spectroscopy of the Primary Photochemistry of Phytochrome. Biospectroscopy 1997, 3, 421–433 [Google Scholar]
- (24).Mathes T; Ravensbergen J; Kloz M; Gleichmann T; Gallagher KD; Woitowich NC; St Peter R; Kovaleva SE; Stojkovic EA; Kennis JTM Femto- to Microsecond Photodynamics of an Unusual Bacteriophytochrome. J. Phys. Chem. Lett 2015, 6, 239–243. [DOI] [PubMed] [Google Scholar]
- (25).Zhong D; Diau EW-G; Bernhardt TM; De Feyter S; Roberts JD; Zewail AH Femtosecond Dynamics of Valence-Bond Isomers of Azines: Transition States and Conical Intersections. Chem. Phys. Lett 1998, 298, 129–140. [Google Scholar]
- (26).Zhuang X; Wang J; Lan Z Tracking of the Molecular Motion in the Primary Event of Photoinduced Reactions of a Phytochromobilin Model. J. Phys. Chem. B 2013, 117, 15976–15986. [DOI] [PubMed] [Google Scholar]
- (27).Falklöf O; Durbeej B Steric Effects Govern the Photoactivation of Phytochromes. Chemphyschem 2016, 17, 954–957. [DOI] [PubMed] [Google Scholar]
- (28).Singer P; Wörner S; Lamparter T; Diller R Spectroscopic Investigation on the Primary Photoreaction of Bathy Phytochrome Agp2-Pr of Agrobacterium fabrum: Isomerization in a pH-dependent H-bond Network. Chemphyschem 2016, 17, 1288–1297. [DOI] [PubMed] [Google Scholar]
- (29).Bizimana LA; Farfan CA; Brazard J; Turner DB E to Z Photoisomerization of Phytochrome Cph1 Delta Exceeds the Born-Oppenheimer Adiabatic Limit. J. Phys. Chem. Lett 2019, 10, 3550–3556. [DOI] [PubMed] [Google Scholar]
- (30).Mueller MG; Lindner I; Martin I; Gärtner W; Holzwarth AR Femtosecond Kinetics of Photo-conversion of the Higher Plant Photoreceptor Phytochrome Carrying Native and Modified Chromophores. Biophys. J 2008, 94, 4370–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chosrowjan H; Taniguchi S; Mataga N; Unno M; Yamauchi S; Hamada N; Kumauchi M; Tokunago F Low-Frequency Vibrations and Their Role in Ultrafast Photoisomerization Reaction Dynamics of Photoactive Yellow Protein. J. Phys. Chem. B 2004, 108, 2686–2698. [Google Scholar]
- (32).Arpin PC; Turner DB; McClure SD; Jumper CC; Mirkovic T; Challa JR; Lee J; Teng CY; Green BR; Wilk KE; Curmi PMG; Hoef-Emden K; McCamant DW; Scholes GD Spectroscopic Studies of Cryptophyte Light Harvesting Proteins: Vibrations and Coherent Oscillations. J. Phys. Chem. B 2015, 119, 10025–10034. [DOI] [PubMed] [Google Scholar]
- (33).Slavov C; Xu X; Zhao K; Gärtner W; Wachtveitl J Detailed Insight into the Ultrafast Photoconversion of the Cyanobacteriochrome Slr1393 from Synechocystis sp. Biochim. Biophys. Acta, Bioenerg 2015, 1847, 1335–1344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


