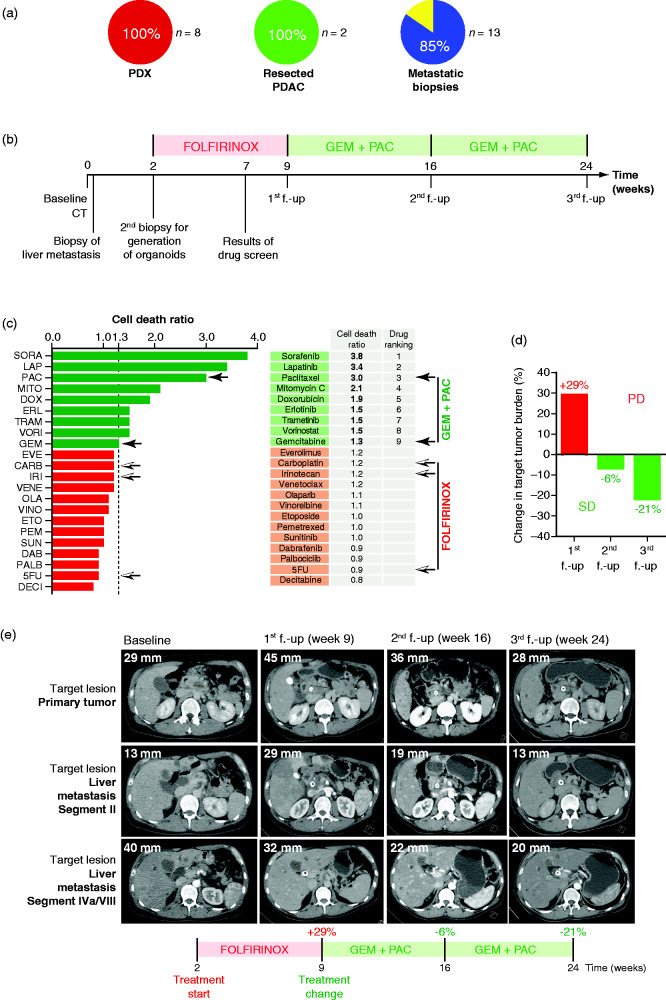
Figure 5.
Organoid-based tailored therapies. (A) Take rate of pancreatic ductal organoid (PDO) isolation from PDX (n = 8), resected primary pancreatic ductal adenocarcinoma (PDAC) (n = 2) and ultrasound-guided biopsy of the primary cancer or liver metastases (n = 13). (B) Scheme of the course of treatment of the patient. (C) Twenty-two drug viability assay screening performed on patient PDO (one dose per drug). Black arrows show components of GEM + PAC therapy. Black-and-white arrows show components of FOLFIRINOX therapy. (D) Waterfall plot showing percent change in tumor burden determined based on RECIST 1.1. criteria at first follow-up (f.-up) with an increase of 29% corresponding to progressive disease (PD). At second f.-up and third f.-up, there was a decrease of, respectively, 6% and 21%, corresponding to stable disease (SD). (E) Representative pictures of CT scan. Shown is the longest dimension of target lesion of the primary tumor and target lesion of a liver metastasis segment II and segment IVa/VIII at baseline (time of initial diagnosis), at first f.-up (week 9) after treatment with FOLFIRINOX, at second f.-up (week 16) after treatment with gemcitabine/nab-paclitaxel, and at third f.-up (week 24) after continued therapy with gemcitabine/nab-paclitaxel. 5FU: 5-fluorouracil; CARB: carboplatin; DAB: dabrafenib; DECI: decitabine; DOX: doxorubicin; ERL: erlotinib; ETO: etoposide; EVE: everolimus; GEM: gemcitabine; IRI: irinotecan; LAP: lapatinib; MITO: mitomycin C; OLA: olaparib; PAC: paclitaxel; PALB: palbociclib; PEM: pemetrexed; SORA: sorafenib; SUN: sunitinib; TRAM: trametinib; VENE: venetoclax; VINO: vinorelbine tartrate; VORI: vorinostat.