Figure 2. Screening a kinase inhibitor library for p38γ inhibitors led to the selection of F7/PIK75.
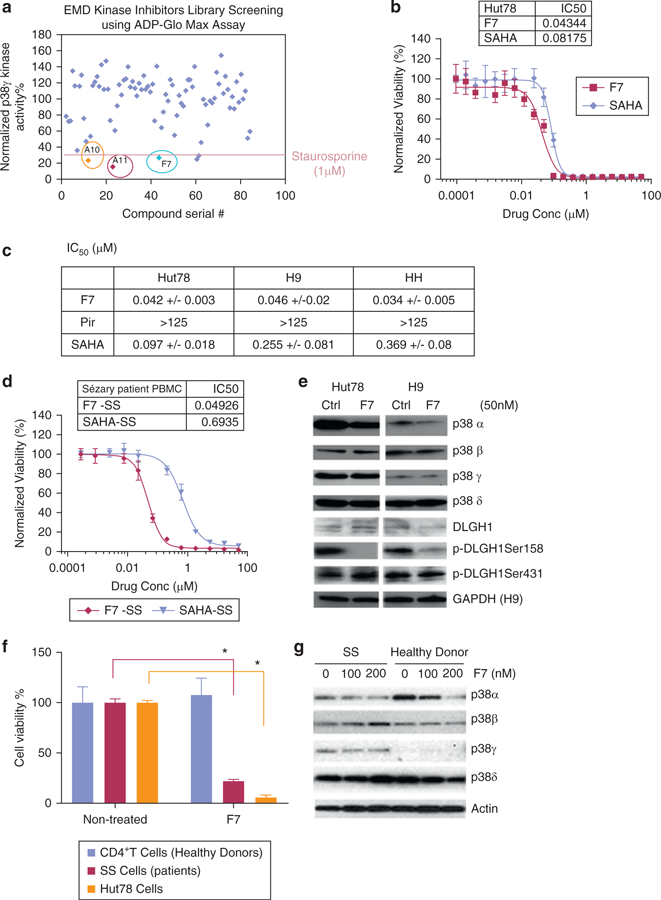
(a) A 260 kinase inhibitor library (EMD Biosciences, San Diego, CA) was screened for p38γ activity using ADP-Glo Max Assay (normalized to DMSO control). The three most potent candidates (A10, A11, and F7) are indicated. 1 μmol/L staurosporine was used as an internal positive control. (b) Data represented are normalized to DMSO control in Hut78 cells treated with varying concentrations of F7/PIK75 or SAHA (an FDA-approved drug for treatment of CTCL, used as a control). Inset table shows calculated IC50 values (μmol/L). (c) Determination of IC50 (μmol/L) in Hut78, H9, and HH cells treated with F7/PIK75, pirfenidone (Pir), or SAHA for 72 hours. *p < 0.05. (d) Cell viability was normalized to DMSO control in PBMCs isolated from SS patients and treated with varying concentrations of F7/PIK75 or SAHA for 72 hours. Inset table shows calculated IC50 values (μmol/L). *p < 0.05. (e) Western blot was used to visualize protein expression of indicated p38 isoforms, phosphorylated DLGH1 Ser158 and Ser431, and actin (loading control) in Hut78 and H9 CTCL cells treated with 50 nmol/L F7/PIK75, using indicated antibodies. (f) Cell viability was measured in CD4+ T cells from healthy donors (n = 2) and SS patient cells (n = 2) treated with 100 nmol/L of F7/PIK75 or DMSO control. Data presented as normalized to untreated controls. (g) Western blot for CD4+ T cells from a healthy donor or an SS patient treated with F7/PIK75 (100 nmol/L or 200 nmol/L) or DMSO control to indicate expression of p38 isoforms and actin as the loading control. All experiments were repeated in three independent experiments, and data represented are the average of triplicate experiments. CellTiterGlo Cell Viability Assay (Promega, Madison, WI) was used to measure viability in b–d. EMD, EMD Biosciences (San Diego, CA); FDA, US Food and Drug Administration; IC50, half maximal inhibitory concentration; M, mol/L; SS, Sézary syndrome.
