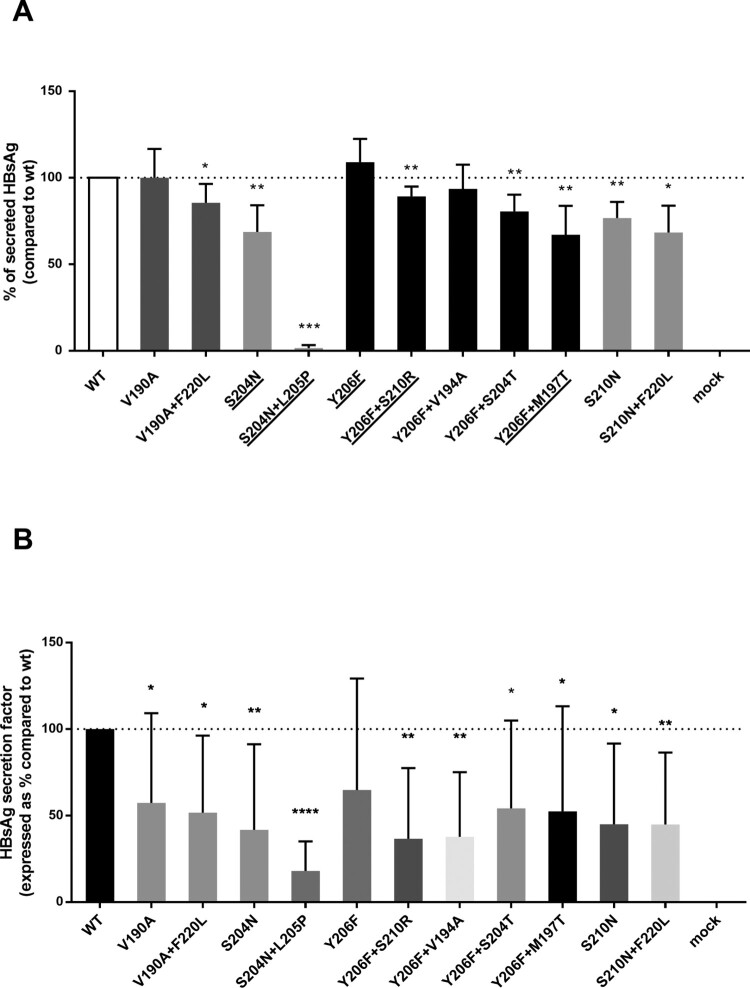
Figure 4.
In vitro impact of HBsAg C-terminus mutations on HBsAg quantification. A plasmid encoding the small HBsAg linked to a streptavidin-tag version II at N-terminus was used to transfect the HepG2 cells. (A) The amount of strep-tagged HBsAg released in culture supernatants was then quantified using a specifically-designed ELISA capable to recognize the Strep-tag linked to the HBsAg. For each mutant, the amount of strep-tagged HBsAg released in supernatants of HepG2 cell cultures was expressed as percentage, considering the amount of the wild-type strep-tagged HBsAg as 100%. Results represent the mean values (+/- standard deviation) of 3 independent experiments, each led in duplicate. * indicates P values ranging from 0.05 to 0.01, ** P values from 0.01 to 0.001 and *** P values <0.001 compared to wild-type. For the underlined pairs of mutations HBsAg release was significant also compared to the corresponding single mutation S204N (P<0.001) or Y206F (P=0.007, P=0.001 and <0.0001, respectively). (B) For each mutant, the HBsAg secretion factor was measured as the ratio between extracellular and intracellular strep-tagged HBsAg in HepG2 cells. The amount of extracellular and intracellular strep-tagged HBsAg was measured by the LIAISON® XL murex HBsAg Quant assay (DiaSorin, Italy). HBsAg secretion factor of mutants was expressed as percentage, considering the amount of the wild-type strep-tagged HBsAg as 100%. Results represent the mean values (+/- standard deviation) of 3 independent experiments, each led in duplicate. * indicates P values ranging from 0.05 to 0.01, ** P values from 0.01 to 0.001, *** P values from 0.001 to 0.0001 and **** P values <0.0001 compared to wild-type.