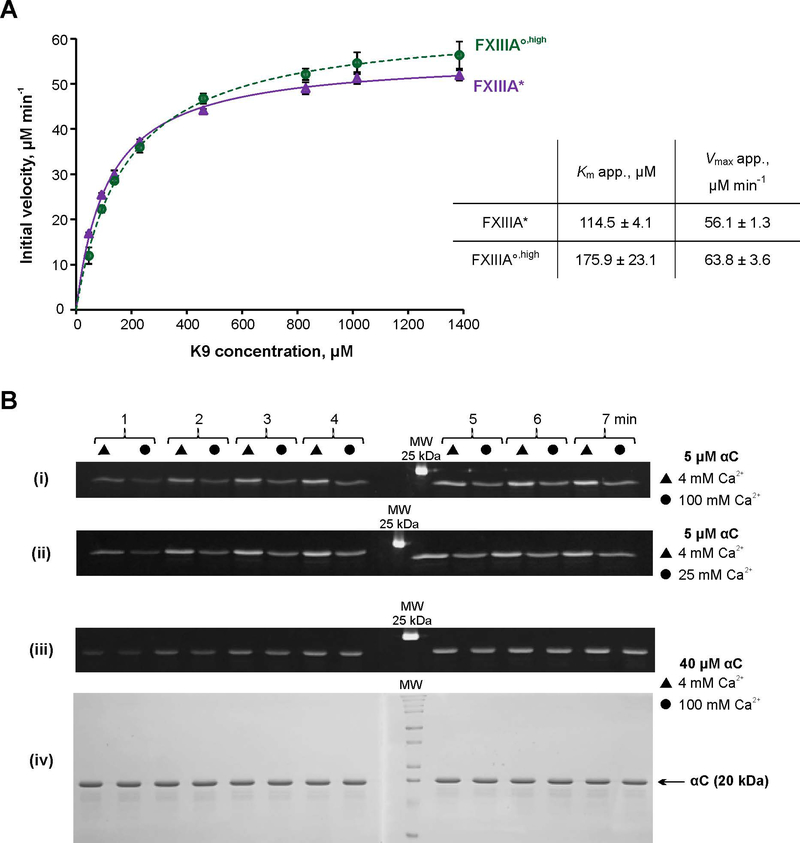
Fig. 4. Transglutaminase activity of FXIIIA under different conditions.
A – Spectrophotometric kinetic assay. Glutamine donor K9 peptide was preincubated with the lysine mimic DMPDA at 37 °C for 5 min, and the crosslinking reaction was initiated by addition of 1 μM FXIIIA°,high (green circle) or FXIIIA* (purple circle). The reaction was followed by an increase in absorbance at 278 nm due to formation of the anilide crosslinked product. The plot represents Michaelis-Menten fits of the initial reaction velocities as function of the K9 concentration. Apparent Km and Vmax resulting from the fits are shown in the table insert. Values are presented as mean ± SD (N=3).
B – MDC crosslinking SDS-PAGE based assay. Recombinant fibrinogen αC (233–425) was preincubated with 1 mM MDC at 37 °C for 5 min. The crosslinking reaction was initiated by addition of 100 nM FXIIIA°,high or FXIIIA*. Reaction aliquots were withdrawn at 1 – 7 min and quenched by addition of reducing sample buffer and boiling. The time point samples were loaded on 15% SDS-PAGE side by side for FXIIIA* (triangles) and FXIIIA°,high (circles). Two gels were run (1 – 4 and 5 – 7 min time points). The gels were aligned and photographed under UV light (panels i – iii). Panel iv – Coomassie Blue-stained gel pair from panel iii demonstrating absence of αC–αC conjugation. FXIIIA* was always preactivated in the presence of 4 mM CaCl2, and FXIIIA°,high was preactivated in the presence of 100 mM CaCl2. Concentrations of αC and CaCl2 in the crosslinking reaction mix are annotated on the right. The different MDC assay series were performed three independent times.