Figure 3.
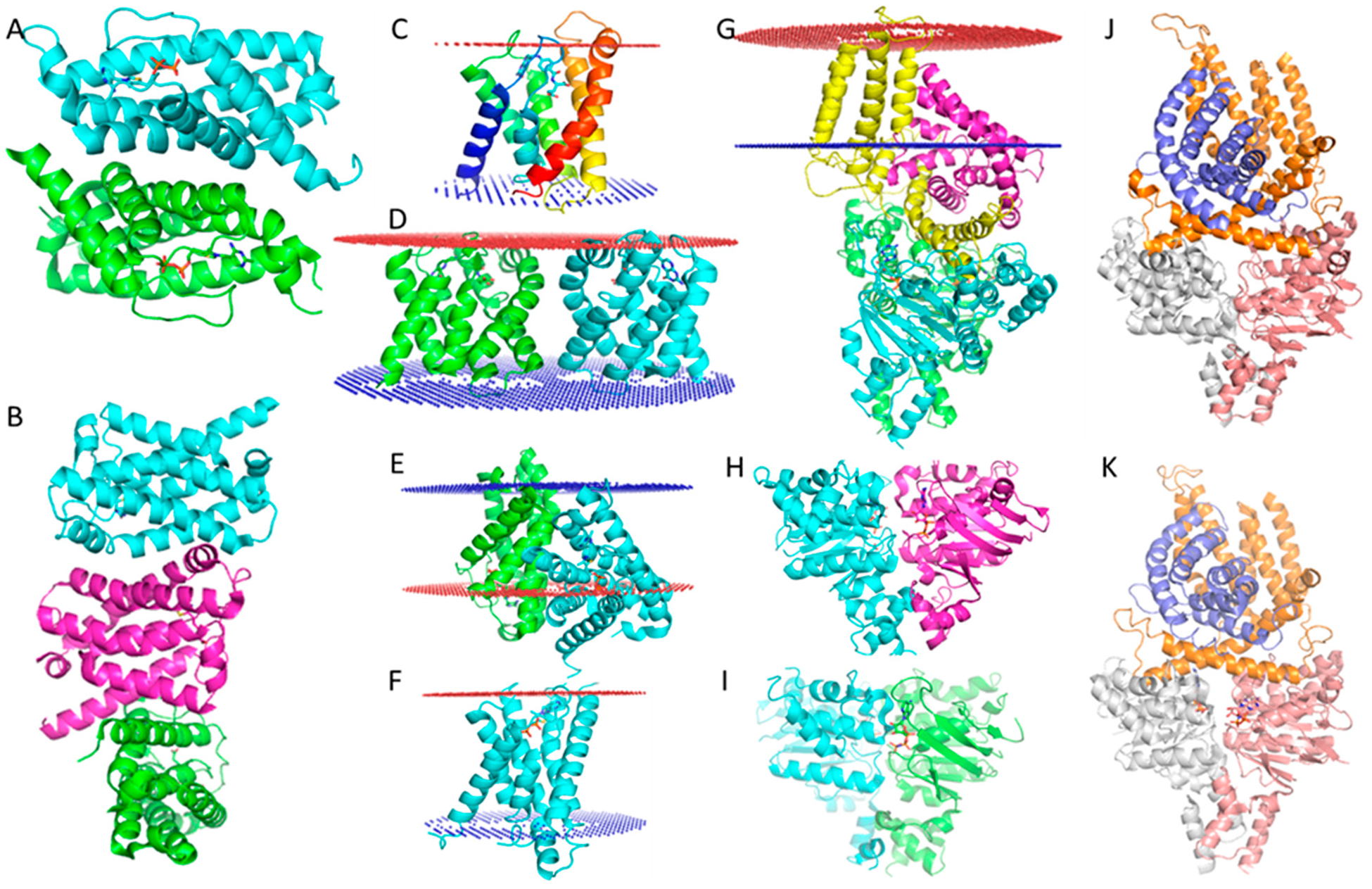
Crystal structures of ECF type of ABC transporter. (A). Crystal structure of the S component of the ECF type ThiT transporter. In the asymmetric unit, two monomers formed an artificial dimer caused by the lipid environment being damaged by detergents. (B). Crystal structure of the ECF type Folate transporter S component. Three S subunits associated into an artificial trimer through two very different dimeric interfaces because of detergent damage to the lipid environment. (C). Transmembrane region analysis with PPM of one S component of the folate transporter. (D). Transmembrane region analysis with PPM of an artificial S component dimer of the folate transporter. The relative orientation of the same S component when analyzed as a monomer is dramatically different from the analysis of the artificial dimer; in the artificial dimer the St component is tilted about 45°. (E). Transmembrane region analysis of ThiT artificial dimer. In this analysis, one S subunit has a normal orientation, however, the other subunit is almost parallel to the cell membrane and strikingly almost half of the S component is outside of the cell membrane. (F). Transmembrane region analysis of a single S component of ThiT, displaying one parallel S component in the same orientation as seen in Figure 3E; however, in the single S component analysis, we see the S component displaying a normal orientation. (G). PPM analysis of the transmembrane region of an ECF type ABC transporter. The T component is in a normal orientation within the calculated transmembrane region; however, the S component is parallel to the transmembrane region. This situation is strikingly similar to the situation displayed in Figure E. Since the dimer in Figure E is an artifact caused by detergent, the evidence suggests the crystallographic structure of the ECF transporter complex is an artifact. (H). ATPase dimer in an open state conformation without ATP or AMPPNP binding. (I). ATPase dimer in a closed state conformation with AMPPNP binding. Binding of AMPPNP induces the closed state conformation of the dimer. (J). ECF transporter complex apo state without ATP or AMPPNP binding to the ATPase domain, ATPase is in an open state conformation. (K). ECF transporter complexed with AMPPNP binding to the ATPase domain, however, the ATPase is still in an open state conformation. This is controversial since the ATPase binding to AMPPNP should induce a closed state conformation. The reason could be that the artificial complex is structurally and functionally aberrant, so AMPPNP could not induce the expected conformational change.
