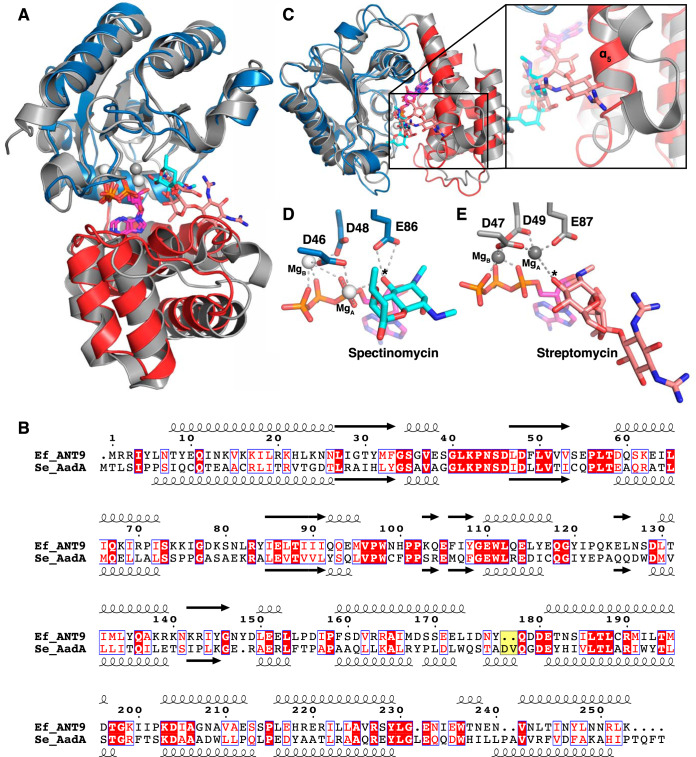
FIG 3.
Comparison of ANT(9) and AadA. (A) Superposition of the N-terminal domain of ANT(9)-ATP-spc (colors as in Fig. 2) with AadA in complex with ATP and streptomycin (15) (gray; streptomycin in salmon). (B) Structure-guided sequence alignment of E. faecalis ANT(9) with S. enterica AadA (15, 42). The characteristic Asp-Val insertion in AadA is highlighted in yellow. (C) The straight α5 in ANT(9) would clash with streptomycin. AadA shows a kink in helix α5, and the insertion in the α5-α6 loop forms a short helix to accommodate streptomycin (salmon). The view is perpendicular to that in panel A. (D and E) Comparison of active-site and magnesium coordination in ANT(9)-ATP-spc (D) and AadA-ATP-sry (E). The asterisk indicates the substrate hydroxyl to be modified.