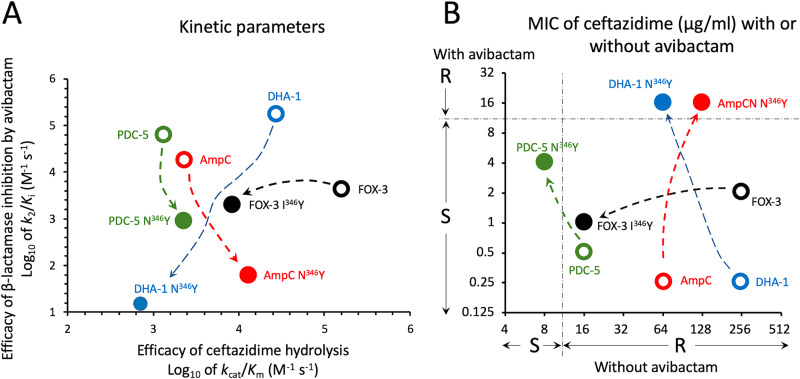
FIG 1.
Impact of critical amino acid substitutions on the kinetic parameters for ceftazidime hydrolysis and β-lactamase inhibition by avibactam (A) and consequences of these modifications on the MICs of ceftazidime alone or in combination with avibactam (B). For AmpCcloacae (AmpC), a modest increase in the efficacy of ceftazidime hydrolysis results in a modest increase in the MIC of ceftazidime. In combination with a large decrease in the efficacy of avibactam, this leads to resistance to the ceftazidime-avibactam combination (MIC = 16 μg/ml). The N346I substitution in DHA-1 is associated with the same level of resistance to the combination, as a reduction in the efficacy of ceftazidime hydrolysis is compensated by a very large decrease in the efficacy of avibactam. For PDC-5, the reduction in the efficacy of avibactam is relatively modest. This leads to an increase in the level of resistance to ceftazidime-avibactam but resistance to the combination is not achieved, despite a relatively low efficacy of ceftazidime hydrolysis. For Fox-3, the I346Y substitution impairs the efficacy of ceftazidime hydrolysis, leading to a large decrease in the ceftazidime MIC. However, strains producing FOX-3 and FOX-3 I346Y remain susceptible to the ceftazidime-avibactam combination since the substitution has only a moderate impact on the efficacy of avibactam. S, I, and R, susceptible, intermediary, and resistant according to EUCAST breakpoint values for P. aeruginosa.