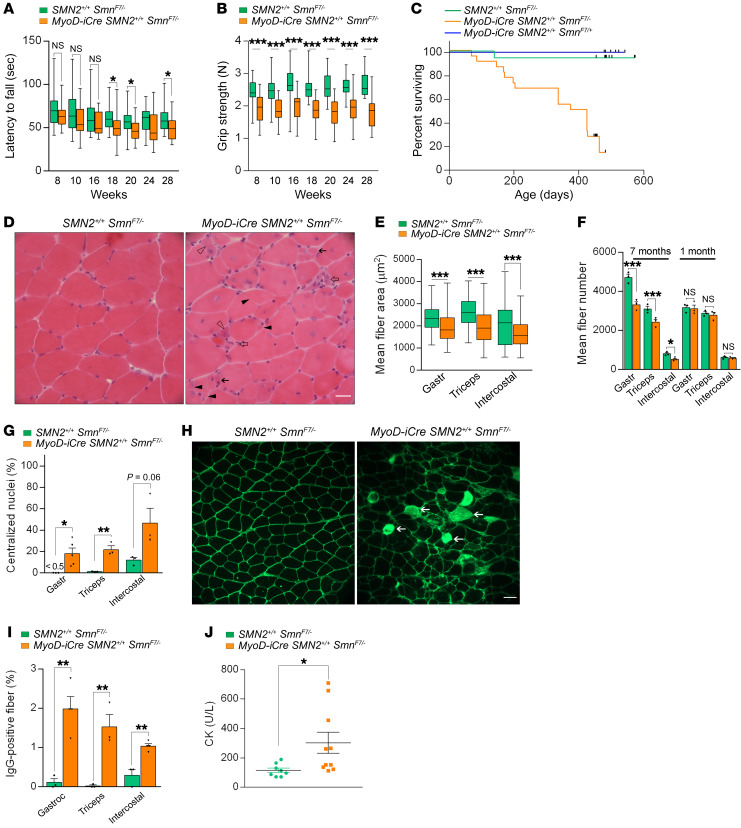
Figure 6. Late-onset disease in MyoD-iCre SmnF7/– mutants bearing 2 SMN2 copies.
Results of (A) rotarod and (B) grip strength tests demonstrated the late-onset, disease-causing effects of selectively depleting SMN in skeletal muscle; t test, n ≥ 25 mice of each genotype. (C) Kaplan-Meier survival curves depict reduced lifespan of mutants. P < 0.0001 between mutants and the 2 sets of controls; P > 0.05 between the 2 sets of controls, log-rank test. (D) Transverse sections from 6- to 7-month-old mutant and control gastrocnemius muscles illustrate the presence of pathology in mutants. Hypotrophic fibers (solid arrows), regenerating fibers with cytoplasmic basophilia (open arrowhead), split fibers (solid arrowheads), or degenerating fibers (open arrows) are shown. Scale bar: 25 μm. Graphs represent (E) fiber sizes and (F) fiber numbers in muscles from 7-month-old mutants and controls; t test, n ≥ 500 fibers from n ≥ 3 mice of each genotype. (G) Estimates of central nuclei in muscles of 7-month-old mutants or controls; t test, n ≥ 1000 fibers from n ≥ 3 mice of each genotype. (H) Transverse sections of gastrocnemius muscles from 7-month-old mutant and control mice depicting damaged IgG-positive myofibers (arrows). Scale bar: 50 μm. (I) Quantification of damaged fibers from the previous experiment; t test, n ≥ 300 fibers from n ≥ 3 mice of each genotype. (J) Quantified results of serum CK values from 7-month-old mutant and control mice; t test, n ≥ 8 mice of each cohort. *P < 0.05; **P < 0.01; ***P < 0.001.