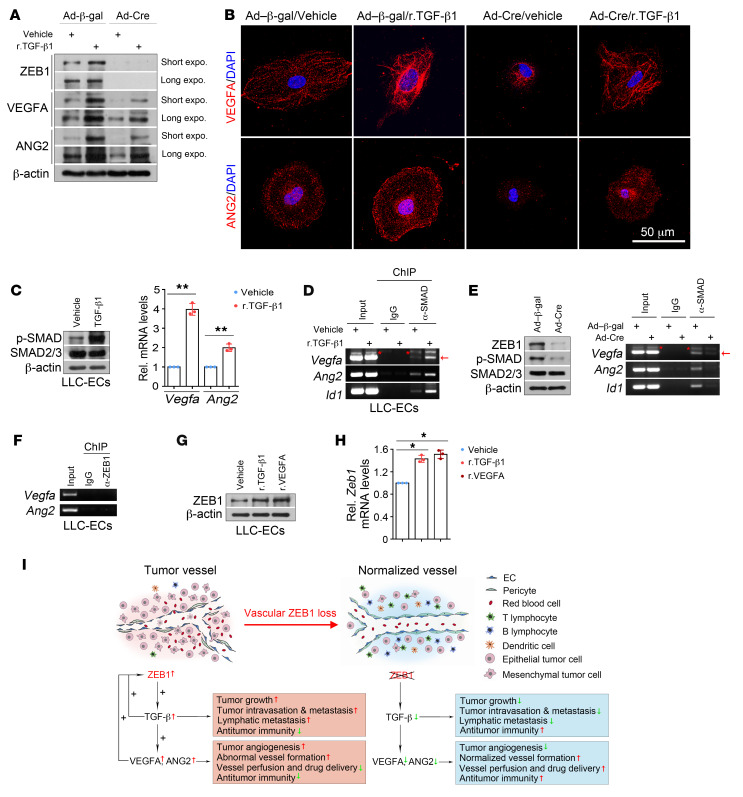
Figure 13. ZEB1/TGF-β/SMAD signaling transactivates Vegfa and Ang2 genes in LLC tumor ECs.
(A) Immunoblot analysis of control and ZEB1-deleted LLC-ECs that were treated with vehicle or 2 ng/mL r.TGF-β1 protein for 3 hours. (B) Immunofluorescence analysis of VEGFA (top) and ANG2 (bottom) expression in the indicated cells as described in A. Nuclei, DAPI (blue). (C) Immunoblot (left) and qRT-PCR (right) analyses of vehicle- or r.TGF-β1–treated LLC-ECs for p-SMAD2/3 and SMAD2/3 protein expression and Vegfa and Ang2 mRNA expression (n = 3 independent experiments). (D) ChIP-PCR for assessing SMAD2/3 level on the promoters of Vegfa, Ang2, and Id1 in vehicle- and r.TGF-β1–treated LLC-ECs (n = 3 independent experiments). Around 5 × 106 LLC-ECs were used for each ChIP-PCR experiment. (E) ChIP-PCR for assessing SMAD2/3 level on the promoters of Vegfa, Ang2, and Id1 in control and ZEB1-deleted LLC-ECs (n = 3 independent experiments). Left panel: immunoblot analysis for confirming reduced ZEB1 and p-SMAD2/3 expression in ZEB1-deleted LLC-ECs. (F) ChIP-PCR showing no binding of ZEB1 on the proximal promoters of Vegfa and Ang2 in LLC-ECs (n = 3 independent experiments). (G and H) Immunoblotting (G) and qRT-PCR (H) for assessing ZEB1 protein and mRNA expression in LLC-ECs treated with vehicle, r.TGF-β1 (2 ng/mL, 3 hours), or r.VEGFA (20 ng/mL, 18 hours) (n = 3 independent experiments). (I) Schematic diagram showing the effect of vascular ZEB1 loss on tumor growth and metastasis, tumor angiogenesis, vascular functionality and integrity, and tumor immune microenvironment. All data are represented as mean ± SD. *P < 0.05; **P < 0.01. Differences were tested using unpaired 2-sided Student’s t test (C) and 1-way ANOVA with Tukey’s post hoc test (H).