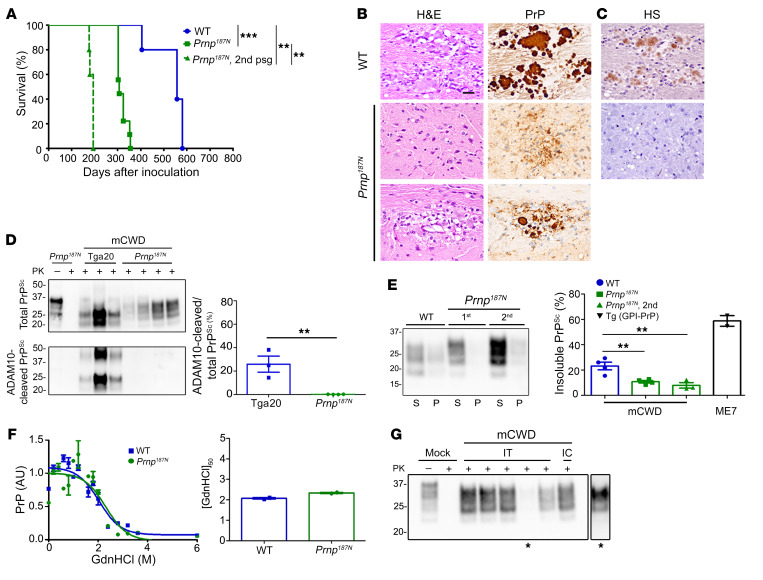
Figure 7. Knockin mice expressing triglycosylated PrP show a reversal in the clinical, histologic, and biochemical phenotype when infected with the plaque-forming prion strain, mCWD.
(A) Survival curves of Prnp187N mice inoculated with mCWD prions show a profound decrease in the survival time as compared with WT mice; n = 5–9/group. (B) The spongiform degeneration is similar in Prnp187N mice as compared with WT mice; however, PrP deposits switched from the typical large, dense plaques in WT mice to primarily diffuse aggregates, with occasional rare small plaques in the corpus callosum (lower panel). (C) HS immunolabelling of the diffuse aggregates or plaque-like structures was not evident in the Prnp187N brain. (D) Western blot reveals that the PrPSc in the Prnp187N brain is no longer composed of ADAM10-cleaved PrPSc that predominated in the original mCWD strain in tga20 mice (overexpressed WT mouse PrP); n = 3–5/mouse strain. (E) Western blot of soluble versus insoluble PK-resistant PrPSc reveals a decrease in insoluble PrPSc in the Prnp187N mice; n = 4–7 WT or Prnp187N mice and n = 2 Tg (GPI-PrP) positive control brain samples. (F) Representative PrPSc stability assay and quantification show that the resistance to unfolding in chaotropes is similar for PrPSc aggregates in the mCWD-infected Prnp187N and WT brain; n = 2/group. (G) Western blot of Prnp187N mice inoculated intratongue (IT) or intracerebrally (IC) with mCWD-passaged Prnp187N brain homogenate shows that all clinically terminal mice (5/5 mice) harbored PrPSc in the brain. *A longer exposure of one sample is shown on the right. **P ≤ 0.01, ***P ≤ 0.001; 1-way ANOVA with Tukey’s test (A and E), unpaired, 2-tailed Student’s t test (D). Scale bar: 100 μm (B and C).