Fig. 2. Generation and in vitro evaluation of anti-HER2 ARC-ADC.
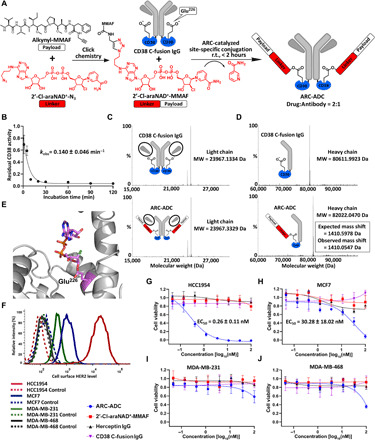
(A) Scheme for generating anti-HER2 ARC-ADC. r.t., room temperature. (B) Conjugation kinetics of drug linker to CD38 C-fusion IgG. 2′-Cl-araNAD+–MMAF (1 mM) was incubated with CD38 C-fusion IgG (10 μM) in 50 mM tris buffer (pH 8.5) for various amounts of time on ice. The residual enzymatic activity determined by fluorescence-based activity assays was plotted as a function of incubation time. (C and D) Mass spectra of light chains (C) and heavy chains (D) for CD38 C-fusion IgG and anti-HER2 ARC-ADC. MW, molecular weight. (E) X-ray structure of human CD38 catalytic domain with 2′-Cl-araNAD+ covalently attached to Glu226 residue. (F) Flow cytometric analysis of HER2 expression for four breast cancer cell lines. (G to J) In vitro cytotoxicity of anti-HER2 ARC-ADC. HCC1954 (G), MCF7 (H), MDA-MB-231 (I), and MDA-MB-468 (J) cells with varied levels of HER2 expression were incubated for 72 hours at 37°C with 5% CO2 in the presence of various concentrations of ARC-ADC, 2′-Cl-araNAD+–MMAF, Herceptin, and CD38 C-fusion IgG. Cell viability was measured by MTT assays. Cells treated with culture media and 5 μM paclitaxel were included as 100% viability and 0% viability controls, respectively.
