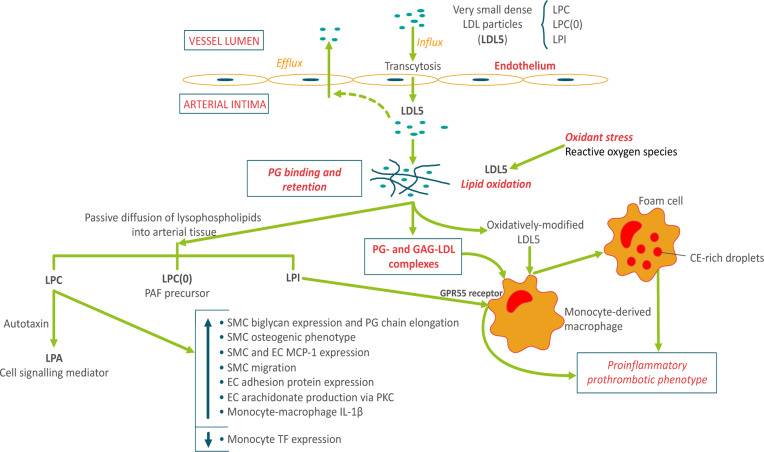
Fig. 5.
Arterial entry and retention of vsdLDL5: relevance to atherogenesis. The small particle size of vsdLDL particles (LDL5) favors enhanced arterial wall entry by endothelial transcytosis (13, 21, 51). These particles are preferentially enriched in three bioactive lysophospholipids: LPC, 9 mol per LDL5 particle; LPC(O), i.e., lyso-PAF, 1 mol per seven LDL5 particles; and LPI, 1 mol per six LDL5 particles, and equally in Lp-PLA2. sdLDLs display elevated binding affinity for proteoglycan components of the extracellular matrix, favoring enhanced intimal retention (21). Complexes of dense LDL5 particles with proteoglycans (PG) and glycosaminoglycans (GAG) are avidly taken up by human monocyte-derived macrophages (HMDMs) (52). Systemic oxidative stress is a characteristic of dyslipidemic MetS subjects (53); LDL5 lipids are highly susceptible to oxidative modification (21). Oxidative modification leads to scavenger receptor uptake in HMDMs with conversion to pro-inflammatory prothrombotic foam cells, key components of atherosclerotic plaques (1, 21). Hydrophilic lysophospholipids [LPC, LPC(O), LPI] may diffuse out from LDL5 particles into the aqueous phase in arterial tissue. LPC can exert a spectrum of biological effects via two pathways: the indirect pathway involving autotaxin-mediated hydrolysis of LPC with formation of lysophosphatidic acid (LPA), a potent cell signaling mediator acting through multiple G-coupled receptors (32, 54); and the direct pathway involving i) stimulation of biglycan expression and PG-chain elongation in vascular smooth muscle cells (VSMCs) with induction of an osteogenic phenotype in these same cells (55, 56), ii) stimulation of monocyte chemoattractant protein-1 (MCP-1) expression in both VSMCs and vascular endothelial cells (ECs) (32), iii) stimulation of VSMC migration (32), iv) induction of adhesion protein expression in ECs and VSMCs (32), v) stimulation of release of arachidonic acid from ECs via the PKC pathway (32), and vi) induction of the production of IL-1β in HMDMs (57). Together these effects of LDL5-associated LPC indicate a key role for these particles in the acute and chronic inflammatory dimensions of atherogenesis (21, 32, 55, 58). By contrast, LPC suppresses tissue factor expression in human monocytes (59). The degree to which LDL5-associated lyso-PAF may be transformed to active pro-inflammatory PAF depends largely on the potential for its acylation by HMDMs in situ (60). Finally, the LPI/GPR55 axis may initiate a wide range of cellular responses, including cytokine and chemokine secretion, cell proliferation and migration, and platelet aggregation (61, 62). Considered together, these findings indicate that the enhanced intimal entry, retention, and oxidation of lysophospholipid-laden LDL5 particles may exert a spectrum of biological actions in arterial tissue, which together favor accelerated atherogenesis in dyslipidemic individuals.