Figure 3.
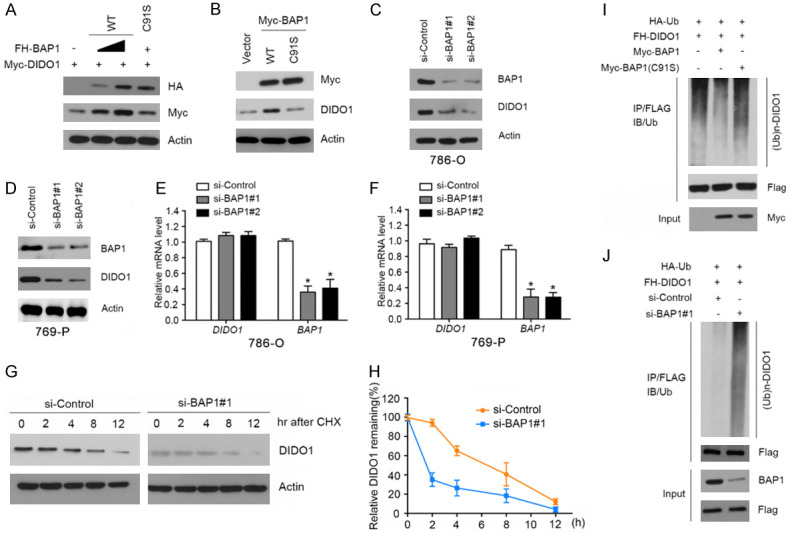
BAP1 stabilizes DIDO1 by de-ubiquitination. A. 293T cells were transfected with Myc-DIDO1 and increasing amounts of FH-BAP1-WT or C91S mutant constructs. 24 h after transfection, cells were harvested for WB analyses. B. 786-O cells were transfected with Myc-BAP1-WT or C91S mutant constructs. 24 h after transfection, cells were harvested for WB analyses. C, D. 786-O and 769-P cells were transfected with the negative control or two independent BAP1 siRNAs, respectively. 48 h after transfection, cells were harvested for WB analyses. E, F. qRT-PCR measurement of the mRNA levels of BAP1 and DIDO1 in BAP1-depleted 786-O and 769-P cells. GAPDH was used for normalization. The mean values (S.D.) of three independent experiments are shown (*, P<0.05). G, H. 786-O cells were transfected with the negative control or BAP1 siRNAs. 48 h after transfection, cells were collected at various times after cycloheximide (CHX) treatment and then were subjected to WB analyses. At each time point, the relative intensities of DIDO1 were first normalized to the intensities of actin and then to the value of the 0-hr time point. Similar results were obtained from two independent experiments. I. HA-Ub and FH-DIDO1 along with Myc-BAP1-WT or C91S constructs were co-transfected into 786-O cells. 24 h after transfection, cells were treated with 20 μM MG132 for 6 h. FH-MDIDO1 protein was immunoprecipitated with anti-FLAG antibody. The ubiquitinated forms of DIDO1 were analyzed by WB with anti-HA antibody. J. BAP1 was depleted by siRNAs in 786-O cells transiently expressing HA-Ub and FH-DIDO1. Cells were treated with 20 μM MG132 for 6 h. FH-DIDO1 protein was immunoprecipitated and subjected to WB analyses.
