Very low dose radiotherapy (4Gy; VLDRT) is an effective treatment for indolent lymphomas and offers the advantage of a shorter course and improved side effect profile compared to full dose RT regimens (24 Gy) [1]. For follicular lymphoma (FL), VLDRT has been generally limited to low grade (1–2) histology. The management of higher grade FL (3A) remains controversial [2,3]; despite a consensus that Grade 3B is an aggressive malignancy akin to diffuse large B cell lymphoma (DLBCL), there is a debate whether grade 3A is better classified as indolent or as aggressive [3–8]. Given the uncertainty, the appropriateness of VLDRT for 3A remains an open question that has not been well studied. The recent multi-institutional ILROG analysis of definitive (≥24Gy) radiotherapy for localized FL included 3A histology and found no significant decrement in terms of disease progression compared to lower grade disease (hazard ratio 0.9, 95% CI 0.50–1.63, p=0.73) [4]. We hypothesize that grade 3A FL may display similarly high radiosensitivity characteristic of Grade 1–2 FL. We thus analyzed 10 consecutive patients with grade 3A or grade 3 not otherwise classified FL who received 2 Gy x 2 fractions as treatment of their disease between 2005–2018 (Table 1). Median age was 71 years (range 52–92) with 7 (70%) females. Response at first post-VLDRT follow-up (median 1.7 months, range of 1.0–4.2) was evaluated by CT (and PET where available) using the Lugano criteria [9]. One patient was evaluated clinically without imaging at 2 months per physician preference. Freedom from local failure (FFLF) was defined as the time from VLDRT to LF and was analyzed per treated RT field. For FFLF, patients were censored at the start of a subsequent therapy if the VLDRT-treated site had not yet progressed. Distant failure (DF) was defined as progression outside of the VLDRT field and freedom from DF (FFDF) was analyzed per patient. FFLF and FFDF were analyzed with Kaplan-Meier using SPSS v25 (IBM, Armonk, NY).
Table 1.
Overview of demographic and treatment characteristics.
| First post VLDRT response assessment | Post-VLDRT follow-up and further treatments | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Sex | Age at VLDRT | Disease state at VLDRT | Lymphoma treatments prior to VLDRT | Duration between most recent prior biopsy and VLDRT (months) | Grade at VLDRT | Initial stage | Stage at VLDRT | Site treated with VLDRT | Max SUV on pre-VLDRT PET | LDH level prior to VLDRT | Months post VLDRT | Mode | Treatment response | Post VLDRT relapse? | Local failure | First out of VLDRT field failure | Additional therapies | Total follow-up (mos) |
| 1 | M | 56 | New diagnosis | none | 1 | 3a | III | III | right submandibular | 14.2 | 91 | 4.2 | CT | CR | Yes | Yes (17.1 mos)*** | 1. Observation | 21.5 | |
| 2 | F | 84 | New diagnosis | none | 3 | 3a | II | II | multifocal head and neck | 17.6 | n/a | 1.3 | PET-CT | PR | No | 11.0 | |||
| 3 | F | 52 | Post observation | 1. Observation x 7 years | 3 | 3a | III | III | right parotid | 10.9 | 190 | 2.2 | PET-CT | CR | No | 36.6 | |||
| 4 | F | 82 | Post observation | "1. Observation x1.5 years 2. Excisional bx of vulvar mass" | 1 | 3a | IV | IV | vulva and cervix | 6.8 | n/a | 1.6 | PET-CT | PR | Yes | Yes (1.5 mos)** | 1. Additional course of VLDRT to in field and out of field PD 2. Considered RCHOP for subsequent DLBCL transformation but lost to follow-up | 31.4 | |
| 5 | F | 70 | Post observation | "1. Excisional bx of hard palate 2. Observation x 3.5 years" | 1 | 3a | IV | II | right neck | 7.3 | 218 | 1.4 | clinical | CR | No | 27.4 | |||
| 6 | F | 73 | Relapsed | "1. Observation x5 years 2. Rituximab monotherapy 3. R-CHOPx3 cycles with rituximab maintenance 4. R-Bendamustine x 4 cycles" | 6 | 3a | IV | IV | right breast and axilla | 6.1 | 211 | 1.9 | PET-CT | CR | No | 43.4 | |||
| 7 | M | 92 | Relapsed | "1. 3600 to right groin 2. Rituximab monotherapy x2 3. 41.4 Gy to left groin" | 106 | 3 | II | II | right axilla, right neck, mediastinum, left neck | 12.5 | n/a | 1.0 | PET-CT | PR | Yes | Yes(6.3 mos)* | Yes (11.0 mos) | 1. Full dose RT (30 Gy) to initially treated field 2. Rituximab for subsequent out of field progression | 42.7 |
| 8 | F | 53 | Relapsed | "1. CHOP x 4 with high dose cyclophosphamide 2. Rituximab monotherapy 3. VLDRT to R epitrochlear region (low grade FL)" | 1 | 3a | Localized | II | right upper arm nodule, R axilla, R SCV | 8.3 | 249 | 2.3 | PET-CT | CR | No | 15.7 | |||
| 9 | F | 62 | Relapsed | "1. Observation x 2 years 2. Rituximab monotherapy 3. R-Bendaustine x 6 cycles 4. Rituximab monotherapy" | 6 | 3a | III | I | pericardial node | 8.0 | 186 | 2.3 | PET-CT | CR | No | 9.8 | |||
| 10 | M | 71 | Relapsed | "1. CHOP --> R-CHOP 2. ICE 3. Rituximab monotherapy 4. Ibritumomab tiuxetan 5. Epitholone" | 25 | 3a | I | IV | right inguinopelvic | n/a | 212 | 1.1 | PET-CT | PR | Yes | Yes (3.3 mos) | Yes (1.1 mos) | 1. VLDRT to two additional fields 2. Steroids | 7.3 |
Fine needle aspiration performed of VLDRT-treated site and cytology positive for malignant cells
First distant progression was not biopsied prior to additional RT but subsequent progression of an additional distant site which developed ~3 years after second course of VLDRT was biopsied as tranformed FL to DLBCL
Distant progressive site biopsied as FL grade 1-2
The cohort was heterogeneous and reflective of the diverse application of RT in the treatment of indolent lymphomas (Table 1). Two (20%) patients received VLDRT palliatively for newly diagnosed disease, 3 (30%) were treated for progression after initial observation (range 1.5–7 years) and 5 (50%) had relapsed disease. Relapsed patients were heavily pre-treated with a range of 3–5 lines of prior therapy. All but one was PET staged before VLDRT with equal distribution of localized stage I-II (n=5, 50%) and advanced stage III-IV (n=5, 50%). Five (50%) received VLDRT to exclusively nodal sites, 3 (30%) to exclusively extranodal and 2 (20%) to mixed nodal/extranodal. The range of pre-treatment max SUV was 6.1–17.6 with no pathological evidence of transformation prior to VLDRT even in patients whose SUV exceeded 10.
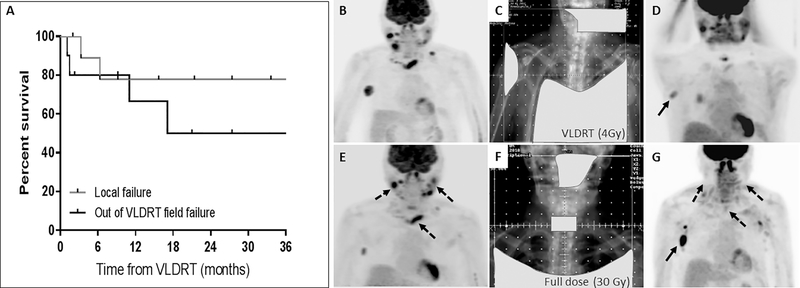
In most cases (80%), the first post-VLDRT assessment was performed using PET/CT. Despite cohort heterogeneity, overall response rate (ORR) at the first post-treatment assessment was 100% (complete response, CR: 6, partial response, PR: 4). There were no adverse events attributable to VLDRT. All seven patients who were symptomatic prior to VLDRT reported clinical improvement at first reassessment. With median follow-up of 24 months (range 7–43), there were 2 LF at 3.3 and 6.3 months, respectively, corresponding to a 1-year FFLF of 78% (95% CI: 36–94%, Figure 1A). Overall, there were 4 DF, corresponding to a 1-year FFDF of 67% (95% CI: 27–88%). Subsequent treatments are detailed in Table 1. Following the initial course of VLDRT, two received additional courses of VLDRT (one with overlapping fields, one non-overlapping) and a third patient received a full dose of RT (30Gy) to the previously VLDRT-treated field following LF. This last patient (Patient 1) highlights a strategy we are increasingly adopting for low grade FL, where patients are offered VLDRT with short-interval radiographic and clinical reassessment with the option to proceed to full dose RT courses for suboptimal responses (Figure 1B–G). Over the analyzed period, we report only one biopsy confirmed transformation to diffuse large B cell lymphoma (DLBCL) which occurred 2.5 years after the initial VLDRT.
Figure 1.
(A) Kaplan Meier curve for LF and DF. (B–G) Illustrative utilization of a sequential palliative RT approach (Patient 7). (B) Pre-VLDRT staging showing recurrent grade 3 A FL predominantly in the right neck, axilla and mediastinum. (C) VLDRT was delivered with conventional fields to a total of 4 Gy. The light gray shapes denote treatment blocks to protect healthy, uninvolved tissue from RT dose. (D) Post VLDRT restaging at 1 month showed very good PR with residual disease in the right axilla (arrow). (E) Restaging imaging at 6 months post VLDRT showed partial in field relapse with progressive disease in the bilateral cervical chains and mediastinum (dashed arrows). (F) The patient then received full dose RT to 30 Gy to the bilateral neck and mediastinum. Given the right axillary disease was stable, it was not included in the field. (G) Post RT imaging 4 months after completion of 30 Gy showed CR in the treated fields and mild progression of the right axillary node which did not require further therapy.
This is, to our knowledge, the first reported dedicated series of high-grade FL treated with VLDRT. We report excellent radiographic and palliative responses across a spectrum of disease states, including several heavily pre-treated patients. VLDRT is advantageous due to significant reductions in normal tissue integral doses. The anticipated acute toxicity from 4Gy is negligible and treatment may be repeated. Additionally, VLDRT is significantly more convenient as it is completed in 2 consecutive days compared to 2–4 weeks for standard, full-dose RT.
Impressive responses after VLDRT in indolent lymphomas prompted the randomized phase III FORT study, designed to test whether 4 Gy was non-inferior to 24 Gy for non-graded FL or marginal zone lymphoma [10]. A total of 614 sites were randomized where the intent of RT was palliative in 60% and curative in the remaining 40%. With median follow up of 26 months, time to LF for 4 Gy was not non-inferior compared to 24 Gy (HR 3.4, 95% CI 2.1–5.6, p<0.0001) and this pattern was observed for both the curative and palliative subgroups. While statistically inferior, the outcomes of 4Gy remained very attractive; ORR was 80% for FL and at 2 years post VLDRT, FFLF remained above 75%.
The authors concluded that while 24 Gy should be standard of care for curable, limited stage disease, full-dose may be an over-treatment for advanced or relapsed FL patients of all histological grades. They suggested that a sequential approach of VLDRT followed by consideration of full dose RT can be applied safely. The logistical benefits of VLDRT and its minimal side effects are attractive for relapsed patients enabling a shorter bridge to additional experimental therapies [11].
Data regarding the use of low dose (4–8 Gy) regimens for aggressive, non-FL histologies (e.g., DLBCL and mantle cell) is very limited, with some small series suggesting ORR may be promising in the context of a palliative strategy [12–15]. VLDRT for aggressive lymphoma has been studied principally in relapsed disease and at this time, remains most appropriate for patients with limited life expectancy. Full dose RT should remain the standard of care for localized 3A patients receiving RT with curative intent.
This study is limited by its small retrospective cohort, but the data are intriguing, suggesting that grade 3A FL has comparable radiosensitivity to low-grade FL which is keeping with other recent series [4,8]. With merely 4 Gy we observed outstanding radiographic responses, local control and associated palliative benefit. Short interval restaging may enable the identification of patients who would benefit from dose escalation, sparing others the unnecessary treatment. These findings support an expanded evaluation of VLDRT in patients with higher-grade FL.
Acknowledgments
Funding: This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. BSI is supported by the Mortimer J. Lacher, MD Lymphoma Fellowship and the Connecticut Cancer Foundation.
Footnotes
Disclosures of Conflict of Interest: The authors of the study have no commercial interests or potential conflicts of interest.
References
- [1].Yahalom J Radiotherapy of Follicular Lymphoma: Updated Role and New Rules. Curr Treat Options Oncol. 2014;15:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vaidyanathan G, Czuczman MS. Follicular lymphoma grade 3: review and updates. Clin Lymphoma Myeloma Leuk. 2014;14:431–435. [DOI] [PubMed] [Google Scholar]
- [3].Zelenetz AD. NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. NCCN; 2019;Version 2.2019. [Google Scholar]
- [4].Brady JL, Binkley MS, Hajj C, et al. Definitive radiotherapy for localized follicular lymphoma staged by 18F-FDG PET-CT: a collaborative study by ILROG. Blood. 2019;133:237–245. [DOI] [PubMed] [Google Scholar]
- [5].Shustik J, Quinn M, Connors JM, et al. Follicular non-Hodgkin lymphoma grades 3A and 3B have a similar outcome and appear incurable with anthracycline-based therapy. Ann Oncol. 2011;22:1164–1169. [DOI] [PubMed] [Google Scholar]
- [6].Ali MM, Rouphail B, Dean RM, et al. Grade 3 Follicular Lymphoma: Outcomes in the Rituximab Era. Blood. 2015;126:1520–1520. [DOI] [PubMed] [Google Scholar]
- [7].Epperla N, Fenske TS, Olteanu H, et al. Outcomes of Grade 3A Follicular Lymphoma: Best Treated As Aggressive or Indolent Lymphoma? Blood. 2016;128:5328–5328. [Google Scholar]
- [8].Ayoub Z, Andraos T, Milgrom SA, et al. Limited stage grade 3 follicular lymphoma patients can experience favorable outcomes with combined modality therapy. Leuk. Lymphoma. 2019;1–9. [DOI] [PubMed] [Google Scholar]
- [9].Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol. 2014;15:457–463. [DOI] [PubMed] [Google Scholar]
- [11].Specht L Radiotherapy for indolent lymphomas: how much is enough? The Lancet Oncology. 2014;15:372–374. [DOI] [PubMed] [Google Scholar]
- [12].Haas RLM, Poortmans P, de Jong D, et al. Effective palliation by low dose local radiotherapy for recurrent and/or chemotherapy refractory non-follicular lymphoma patients. Eur. J. Cancer. 2005;41:1724–1730. [DOI] [PubMed] [Google Scholar]
- [13].Murthy V, Thomas K, Foo K, et al. Efficacy of palliative low-dose involved-field radiation therapy in advanced lymphoma: a phase II study. Clin Lymphoma Myeloma. 2008;8:241–245. [DOI] [PubMed] [Google Scholar]
- [14].Brady JL, Attallah H, Mikhaeel NG. Outcome of Low-Dose Palliative Radiation Therapy in Relapsed or Refractory High-Grade Non-Hodgkin Lymphoma. International Journal of Radiation Oncology • Biology • Physics. 2016;96:E493. [Google Scholar]
- [15].Tanaka O, Oguchi M, Iida T, et al. Low dose palliative radiotherapy for refractory aggressive lymphoma. Rep Pract Oncol Radiother. 2016;21:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]