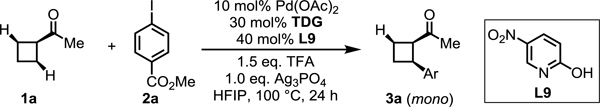
Table 3.
Transient directing group evaluation for enantioselective β-C(sp3)‒H Arylation of cyclobutyl ketones[a,b]
 |
|---|
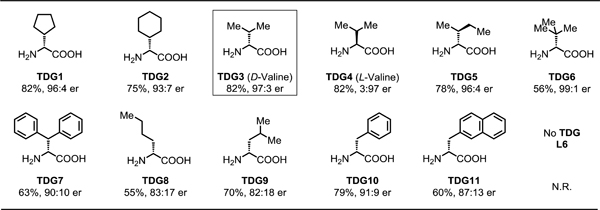 |
Conditions: 1a (0.2 mmol, 2.0 equiv), methyl 4-iodobenzoate (1.0 equiv), Pd(OAc)2 (10 mol %), TDG (30 mol %), L9 (40 mol %), TFA (1.5 equiv), Ag3PO4 (1.0 equiv), HFIP (0.6 mL), 100 °C, under air, 24 h.
Yield determined by 1H NMR analysis of the crude product using CH2Br2 as internal standard.
