Abstract
Purpose
To evaluate the safety and efficacy of methanol extract of Berberis integerrima root on type 2 diabetes compared to metformin.
Methods
In a parallel triple blind clinical trial, 80 type 2 diabetic patients,, were randomized into two groups (treated with Berberis integerrima root, 480 mg (oral), compared to control group treated with metformin 1000 mg daily). Efficacy was evaluated by fasting and prandial glucose and HbA1c and side effects confirmed by physical examination, biology and hematology tests and urinalysis on days 15, 45 and 90. They were followed for 3 months.
Results
Two hundred and eighteen patients were recruited and 80 (55female and 25 male) patients randomized in two groups and 60 patient were analysed. The mean age of patients was 51.8 ± 9.3 and 46.5 ± 10 in the experimental (Berberis integerrima) and control (metformin) groups respectively (P = 0.02). The mean HbA1c at baseline was 8.1 ± 1.6% and 7.9 ± 1.6% for B. integerrima and metformin group respectively (P = 0.53), and there was no significant difference between the two groups (7.5 vs. 7.2) after 3 months (P = 0.34).
Weight loss was observed in both groups compared to baseline.
No adverse event led to preventing the study was reported.
Conclusion
Berberis integerrima root not only was effective as much as metformin in reducing blood glucose and controlling type 2 diabetes but also, no specific side effect was reported (in short term).So, it might be an effective and safe complementary therapy in diabetic patients.
Iranian Research and Clinical Trial (IRCT) registeration number; 201,207,191,774 N5.
Funding: Vice chancellor for research, Physiology Research Center of Kerman University of Medical Sciences and the Exir pharmaceutical company.
Keywords: Berberis integerrima, Complementary medicine, Diabetes mellitus, Zarch, Herbal, Blood glucose
Introduction
Diabetes mellitus is a common disease worldwide and our country type 2 diabetes accounts for 90% of all cases of diabetes and the disease’s incidence in Kerman province is 7.9% [1].
This disease is the most common cause of renal failure and non-traumatic lower limb amputation. Given the foregoing, the diagnosis and treatment of diabetic patients has traditionally been one of the most important goals of medicine.
Currently, to control blood glucose in type 2 diabetes mellitus, in addition to dietary, exercise, and weight loss, different drugs with multiple effects in reducing blood glucose are used [2].The use of alternative medicine, especially herbal medicine as the most popular branches of alternative treatment is of great importance; now more than 800 species have been recorded in the treatment of diabetes. [3] One of the herbals used by diabetic patients to reduce blood glucose is berry root known as Zarch (the local name) and scientific name Berberis integerrima. Anti-depression and anti-diarrhea effects of Berberis aristata roots have been proved in animal studies [4, 5].The root of Berberis aristata has been administered as anti arrhythmics drug (CardioRhythm) in ventricular arrhythmias induced by lack of oxygen after myocardial ischemic injury. In addition, the root extract of this plant is used in preparing an anti-inflammatory drug with the brand name D-Flame that is Cox-2 inhibitor [5].Recently, several studies have been conducted showing the main mechanism of the effect of this plant on blood glucose due to certain alkaloid called berberine acted as DPP-4 inhibitors(dipeptidyl peptidase- 4 inhibitor ); as well as other mechanisms such as stimulation of insulin secretion and enhancing peripheral glucose uptake [6, 7].Recently a study showed that Berberry Juice consumption for 8 weeks led to decreased FBS, TC and blood pressure and in T2DM patients. [8] In 2019 a meta analysis showed that the addition of silymarin to berberine is able to improve its positive effect on lipid and glucose metabolism in humans,how ever silymarin per se could exert some additive effects on lipid and glucose parameters. [9] On our best knowledge up to now the effect of Berberis aristata in lowering blood glucose has been shown only in animal studies. In addition of positive effects on blood glucose, it has been effective on reducing cholesterol levels and improving liver and kidney function [10]. Anti hyperglycemic effect of ethanol extract of Berberis aristata root in alloxan-induced diabetic rats was examined in a study and a reduction of serum glucose, cholesterol, and triglycerides level was observed. However, HDL levels were unchanged and in general, it was suggested that the ethanol extract of B. aristata propounds anti-diabetic activity in alloxan induced diabetic rats [11].In many studies the anti-bacterial, anti-platelet, anti-cancer, anti-inflammatory, antipyretic, analgesic and anti-oxidant activity of Berberis aristata extract have been investigated. Recently antimicrobial and biosafety of Berberis aristata root extract was established. [12] As we could find no human research conducting on the medicinal effects of Berberis integerrima on reducing blood glucose, this study investigated the effect of Berberis integerrima on blood glucose (Efficacy) compared with metformin tablet and its complications (Safety) on the kidneys, liver and the coagulation system in a short term in patients with type 2 diabetes.
Materials and methods
We designed an intervention-based, triple-blind clinical trial. The study was approved by Ethics Committee of Kerman University of Medical Sciences; license number 806/68/10, as well as Iranian Research and Clinical Trial (IRCT); 201,207,191,774 N5 recorded number (full trial protocol can be accessed). Informed consent was obtained from all patients. The study population included patients with type 2 diabetes who referred to endocrinology clinic of Afzalipour hospital and met the inclusion criteria as follows:
Patients with type 2 diabetes according to ADA criteria [13], and the age group between 18 and 75 years who received no medication or were only treated with metformin. Exclusion criteria included FBS above 250 treated with insulin, HbA1c greater than 10%, pregnancy, lactation, advanced chronic complications of diabetes, liver, kidney and known neurological diseases, and chronic inflammatory disease (based on examination, questioning or the patient’s previous documentation). From the patients referred to the clinic those who satisfy the inclusion criteria were enrolled. Participants received verbal explanation and written informed consent was obtained. Patients were divided into 2 groups according to the balance blocked randomization. Participants were randomized by a computer-generated list. Patients in each group received same envelopes containing different medications, identified by A, B letters. Both patients and the physicians were blinded to the treatment received. A nurse who was not involved in the care of the patients was responsible for allocation, preparation, and accounting of trial medications. The trial medication and placebo were prepared at a separate site, and then were taken to the clinic every week. The randomization schedule was thus concealed from all the care providers, ward physicians, and other research personnel. The first group (group A) received methanol extract of Berberis integerrima (in the form of capsules 480 mg) orally once a day, after breakfast with placebo of metformin two times a day. The second group (group B) received metformin tablets (500 mg) two times a day with placebo capsules of Berberis aristata, (prepared from starch powder) once daily. Berberis capsules were hand filled and metformin was prepared by Exir company. In both groups diet and exercise were recommended.
Preparation of extract and placebo:
B.integerrima root was collected from deserts surrounding Baft area and verified by an expert botanist. After collecting and separating the roots, they were air- dried (off from direct sunlight) and 1000 g of dried root was crushed by a proper grinder and soaked in 2 l of methanol for 48 h at room temperature. Then, the roots were extracted by maceration with methanol followed by percolation method.
Afterward, the extract was completely concentrated using a vacuum evaporator; the process was followed by lyophilization. The dried extracts were stored in the airtight vials in the refrigerator. The human equivalent dose of B. aristata root in anti arrhythmic drugs is 1 mg daily; not for reducing blood glucose. The maximum amount used in other studies to induce anti-diarrhea and anti-microbial effects in rats, was 10 mg / kg which had not revealed a significant adverse effect. In a study on the hypoglycemic effect of B. aristata on normal and diabetic rats, as 2500 mg/kg didn’t show fatal effects in rats, 1/10 dose; 250 and 500 mg/kg was used. [14]
Since the least amount used in animal studies was 25 mg / kg, and regarding the ratio of human metabolism to rat, given doses of the drug in humans was set as 1/3; 480 mg once a day before breakfast. To prescribe a daily dose, extracts were filled into size zero capsules, and to contribute to the better absorption, hydrophilic filler (Starch) was used. Then, placebo capsules for the control group, exactly the same shape and weight, were also prepared. Metformin and placebo of metformin were provided by Exir Company of Boroujerd City, Iran. B. integerrima plus placebo of metformin were administrated in group A and metformin plus placebo of extract in group B. Patients in each group received same envelopes containing different drugs. Both patients and the physicians were blinded to the treatment received. Tolerability doses for each individual were determined based on the side effects confirmed by physical examination, biology and hematology tests and urinalysis on days 15, 45 and 90.
A brachial vein blood sample was taken for testing:
Fasting plasma glucose (FPG), 2 h post prandial glucose(2HPG), creatinine(cr), Triglycerides (TG), Cholestrol (chol),High Density Lipoprotein(HDL), Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Alkaline phosphatas(Alk), partial thromboplastin time (PTT), prothrombin Time (PT), bilirubin (bil) and complete blood count (CBC) . Also urine analysis (U/A) at different time point: 0, 15, 45 and 90 days of treatment in both groups were performed. To prevent the effects of blood storage on glucose and other parameters, the samples were centrifuged and kept in the refrigerator at −20 °C until transported to the laboratory on the same day.
HbA1c was measured by NycoCard Reader II assay in clinics with about 3 ml of citrated blood. Besides, the HbA1c values were measured at baseline and after 90 days of intervention. The body weight and blood pressure at baseline, day 15, day 45 and day 90 in both groups were measured by trained nurses. To evaluate the safety of the drug, a questionnaire containing questions about drug side effects, including severe hunger, diplopia, numbness, headache, dizziness,, blurred vision, abnormal behavioral (anger and isolation), confusion, ataxia, Xerostomia, abdominal pain, anorexia, constipation, diarrhea, urticaria, rash, palpitation, seizure, melena, nausea, vomiting and sore throat were completed at each visit by the clinic nurses. In addition, physical examination was performed by a physician and any abnormality, in particular: hepatomegaly, splenomegaly, lymphadenopathy, icterus and pale conjunctiva were recorded. During the study, any time the patient wishes not to continue the treatment or if there were any complaints or important clinical symptoms or abnormal examinations and laboratory disorders (including anemia, thrombocytopenia, leukopenia and elevated liver enzymes, greater than 2-fold, PT more than 1.5 fold, hypoglycemia less than 70, lipid disorders, etc). and the detection of side effects justified by a physician, the patient was excluded and replaced by another patient. In addition, patients diagnosed with a disease that required medication were excluded. All the processes were recorded. Due to the anticipated long-term duration of the study, causing loss to follow up, fructosamine, as a marker of blood glucose control was measured in the short term in the second week. We tried this randomized controlled trial conform to the CONSORT statement and flow diagram [15].
Sample size calculations and data analysis
In a study performed with the aim of the effects of metformin on reducing HbA1c [16], HbA1c value was reduced from 7.46 ± 1.3 to 6.9 ± 0.9; representing 0.56 reducing effect of metformin.
Given at least 1 unit reductions in HbA1c and α = 0.05, 1-β = 80% and using two-sample comparison of means formula, the sample size for this study was calculated as follows. For convenience, STATA statistical software was used for determining the sample size:
Estimated sample size for two-sample comparison of means:
Test Ho: m1 = m2, where m1 is the mean in population 1 and m2 is the mean in population 2.
Assumptions:
alpha = 0.0500 (two-sided)
power = 0.8000
m1 = 7.46
m2 = 6.46
sd1 = 1.3
sd2 = 1
n2/n1 = 1.00
Estimated required sample sizes:
n1 = 22
n2 = 22
In each group, given 30% probability of loss, 30 patients were enrolled.
Measures of central tendency; mean ± S.D., and descriptive statistics; absolute and relative frequency, were used. Between groups comparing of FPG and HbA1c at baseline and day 90 was performed using student’s t- test and within groups using paired t-test.
To compare the changes in HbA1c and FPG in each group, repeated measure ANOVA was used, to compare the changes in HbA1c, 2HPG and FPG between groups Generalized Linear Models was used. To describe the development of complications in the groups Crosstab and Chi-Square test was used. Statistical analysis were performed by SPSS 16.
Results
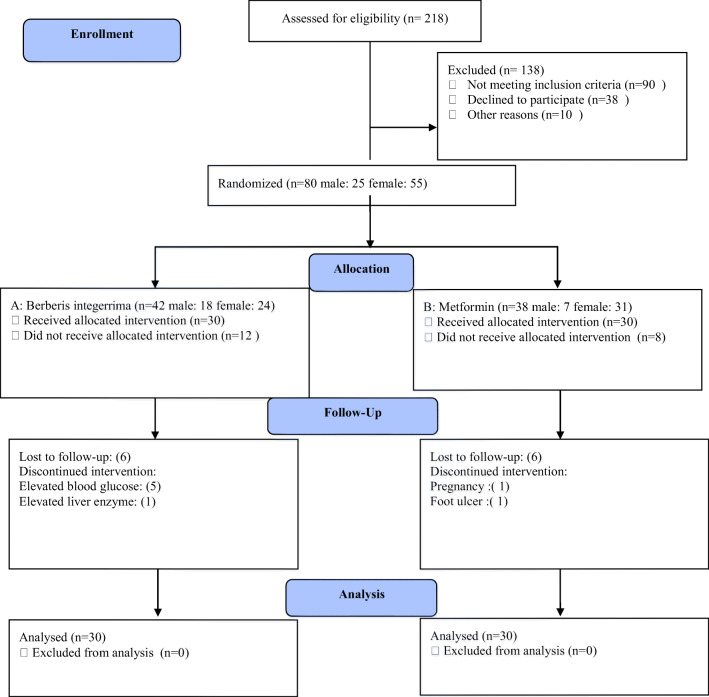
Patient detection began from October 2013. Among 218 patients, contacted following advertising, and recalled to the endocrinology Clinic, 80 patients (55 female and 25 male) who had inclusion criteria were enrolled. In this study,of selected patients 20 cases were excluded and replaced: 12 patients of group A(receiving B. integerrima);one person due to elevated liver enzymes, 5 patients due to lack of blood glucose control and requiring insulin therapy in the first weeks, and 6 patients due to a changing living place and business affairs, and 8 of group B (receiving metformin); a 28-year-old woman because of pregnancy, one person due to foot ulcer and 6 due to the absence (Fig. 1).
Fig. 1-.
CONSORT 2010 Flow Diagram
It should be noted that all the excluded patients were contacted to ensure their health. Finally, 30 patients remained in each group and review was completed during three months, and the data were analyzed.
The mean age of patients was 51.8 ± 9.3 years and (46.5 ± 10 years) for group A and B respectively; patients in the metformin group were slightly younger. Regarding gender distribution 57.1% and 81.6% were female in groups A and B respectively; the difference between the two groups was significant (P = 0.019, Table 1).
Table 1.
Demograhic and laboratory Information of the patients at different times in the two groups
| Day 0 | Day 15 | Day 45 | Day90 | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| group | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | ||
| Age (year) | A | 51.8 | ±9.3 | .02* | ||||||
| B | 46.5 | ±10 | ||||||||
| Weight (kg) | A | 72.5 | ±13.4 | 73.2 | ±14.5 | 70.8 | ±10.8 | 70 | ±9.6 | .43 |
| B | 74.1 | ±13.3 | 73.4 | ±12.9 | 73.0 | ±13.4 | 72.3 | ±10.8 | ||
| Duration (Month) | A | 54 | ±3 | .73 | ||||||
| B | 51 | ±5 | ||||||||
| Height (cm) | A | 163.2 | ±11.5 | .17 | ||||||
| B | 163.2 | ±9.9 | ||||||||
| BMI (Kg/m2) | A | 27.2 | ±4.9 | .22 | ||||||
| B | 27.7 | ±5.3 | ||||||||
| BP systolic (mmHg) | A | 119.0 | ±21.3 | 116.5 | ±16.5 | 115.4 | ±13.5 | 117.9 | ±15.1 | .46 |
| B | 118.6 | ±15.9 | 117.33 | ±15.0 | 117.6 | ±17.4 | 121.08 | ±14.4 | ||
| BP diastolic (mmHg) | A | 74.7 | ±9.6 | 72.2 | ±9.7 | 71.7 | ±7.1 | 77.0 | ±8.0 | .24 |
| B | 77.1 | ±7.3 | 74.3 | ±8.9 | 74.3 | ±8.9 | 74.3 | ±7.8 | ||
| ALT (U/L) | A | 25.9 | ±8.6 | 30.8 | ±17.9 | 27.0 | ±13.0 | 27.2 | ±13.6 | .96 |
| B | 25.7 | ±10.1 | 26.7 | ±14.4 | 25.9 | ±12.2 | 27.1 | ±18.2 | ||
| AST (U/L) | A | 27.2 | ±13.2 | 31.4 | ±13.9 | 30.5 | ±11.3 | 26.8 | ±7.1 | .92 |
| B | 27.2 | ±13.7 | 26.1 | ±6.7 | 24.9 | ±7.1 | 27.1 | ±72.6 | ||
| ALK (U/l) | A | 203 | ±80 | 191 | ±82 | 188 | ±72 | 215 | ±64 | .76 |
| B | 207 | ±82 | 187 | ±59 | 178 | ±54 | 171 | ±721 | ||
| BIL (mg/dl) | A | .8 | ±.6 | .9 | ±.4 | .8 | ±.4 | .7 | ±.3 | .21 |
| B | .9 | ±.8 | .6 | ±.2 | .6 | ±.2 | .6 | ±.2 | ||
| HB (g/dl) | A | 13.8 | ±1.8 | 14.1 | ±2.3 | 14.4 | ±1.8 | 14.3 | ±1.9 | .63 |
| B | 13.8 | ±1.5 | 14.0 | ±1.4 | 13.6 | ±1.3 | 14.0 | ±1.3 | ||
| WBC/μL | A | 6903 | ±1457 | 7024 | ±1673 | 6455 | ±1844 | 6544 | ±253 | .35 |
| B | 7145 | ±7145 | 6894 | ±1814 | 6784 | ±2414 | 6938 | ±346 | ||
| PLT/μL | A | 247,829 | ±9157 | 247,325 | ±10,578 | 240,900 | ± 52,065 | 251,535 | ±13,122 | .45 |
| B | 256,027 | ±9934 | 269,838 | ±11,810 | 276,069 | ±68,068 | 267,824 | ± 69,440 | ||
| PT (g/L) | A | 13.1 | ±.6 | 13.3 | ±.07 | 13.4 | ±.09 | 13.3 | ±.1 | .59 |
| B | 12.7 | ±2 | 13.4 | ±.09 | 13.3 | ±.1 | 13.2 | ±.1 | ||
| PTT (g/L) | A | 34 | ±3 | 33.5 | ±.08 | 33.7 | ±.2 | 33.8 | ±.1 | .31 |
| B | 33 | ±4 | 33.5 | ±.12 | 33.6 | ±.1 | 33.6 | ±.1 | ||
| HbA1c (percent) | A | 8.4 | ±1.7 | 7.5 | ±1.6 | .34 | ||||
| B | 7.8 | ±1.5 | 7.2 | ±1.3 | ||||||
| FPG (mg/dl) | A | 182 | ±51 | 189 | ±69 | 169 | ±57 | 166 | ±60 | .22 |
| B | 167 | ±50 | 153 | ±48 | 156 | ±61 | 148 | ±48 | ||
| 2HPG (mg/dl) | A | 274 | ±103 | 264 | ±121 | 253 | ±104 | 232 | ±95 | .27 |
| B | 261 | ±104 | 221 | ±82 | 226 | ±89 | 207 | ±77 | ||
| Cr (mg/dl) | A | .9 | ±.2 | .7 | ±.1 | .8 | ±.1 | .8 | ±.1 | .14 |
| B | .8 | ±.1 | .8 | ±.2 | .8 | ±.1 | .7 | ±.1 | ||
| TG (mg/dl) | A | 176 | ±122 | 138 | ±71 | 159 | ±90 | 157 | ±91 | .74 |
| B | 170 | ±91 | 147 | ±54 | 139 | ±53 | 150 | ±61 | ||
| Chol (mg/dl) | A | 187 | ±38 | 176 | ±40 | 173 | ±39 | 169 | ±38 | .07± |
| B | 186 | ±39 | 174 | ±51 | 188 | ±31 | 186 | ±30 | ||
| LDL (mg/dl) | A | 113 | ±34 | 111 | ±34 | 103 | ±29 | 103 | ±29 | .18 |
| B | 112 | ±36 | 112 | ±36 | 136 | ±99 | 113 | ±26 | ||
| HDL (mg/dl) | A | 43 | ±9 | 40 | ±9 | 39 | ±7 | 40 | ±11 | .32 |
| B | 44 | ±10 | 42 | ±9 | 45 | ±10 | 43 | ±10 | ||
*Patient in group B were younger
±Cholestrol decreased in group A significantly
At the end of study, mean weight was decreased from 72.9 ± 10.6 to 71.2 ± 10.2 kg in all patients (P = 0.012). Mean weight was 71 ± 10.3 kg at the beginning reaching to 70 ± 9.7 for group A and (P = 0.02) and 74.3 ± 10.9 kg reaching to 72.3 ± 10.9 for group B(P = 0.07); the difference in losing weight was significant in both groups(P = 0.001). Comparing two groups, weight loss was not significantly different (P = 0.40, Fig. 2). Mean systolic (P = 0.46) and diastolic (P = 0.24) blood pressure at different time point in the two groups also showed no significant difference, demonstrating no effect of extract on blood pressure at different times.
Fig. 2.
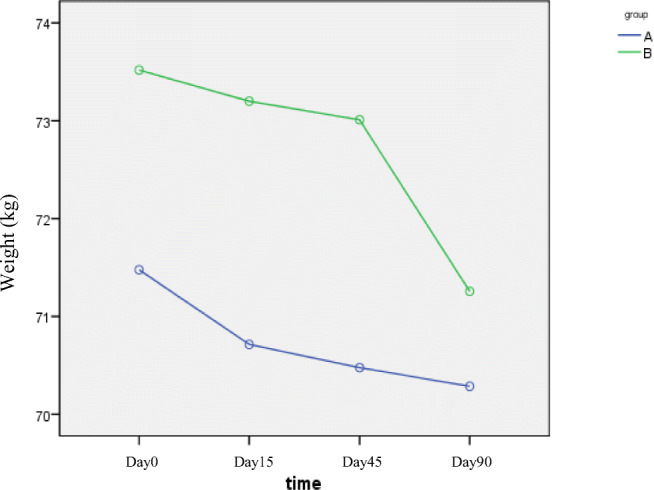
Changes in body weight at different time intervals (A: B. integerrima, B:Metformin). Mean weight was decreased in all patients (P = 0.012). Weight loss was not significantly different (P = 0.43)
In relation to the control of blood glucose, four main indexes: Fasting plasma glucose, 2 h postprandial glucose, fructosamine and HbA1c were examined.
Only fasting plasma glucose (day 15) in group B was significantly lower than group A (189.09 vs. 153.03, P = 0.01), but at other times this difference was not statistically significant indicating that the onset of the effect of metformin was faster than B. integerrima root (Fig. 3).Two hour Postprandial Glucose showed no significant difference between the groups at different times. The maximum effect, especially in group A was in the first month (Fig. 4). FPG and 2 HPG were compared before and after treatment in both groups that significantly reduced (P = 0.01, P = 0.018).
Fig. 3.
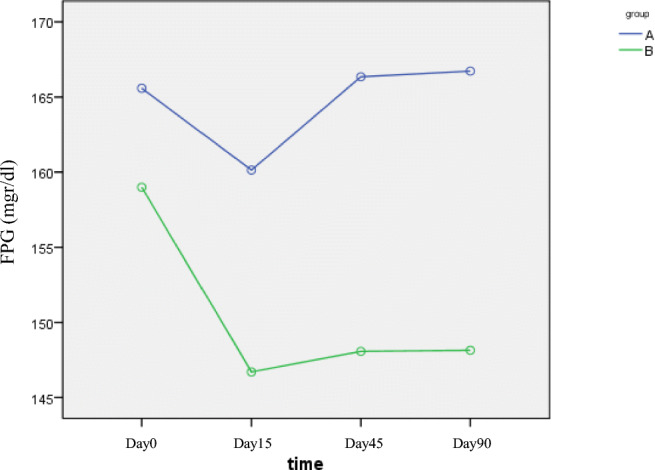
The FPG changes during different time intervals (A: B. integerrima, B: Metformin). The onset of the effect of metformin was faster than B. integerrima root. (P = 0.01)
Fig. 4.
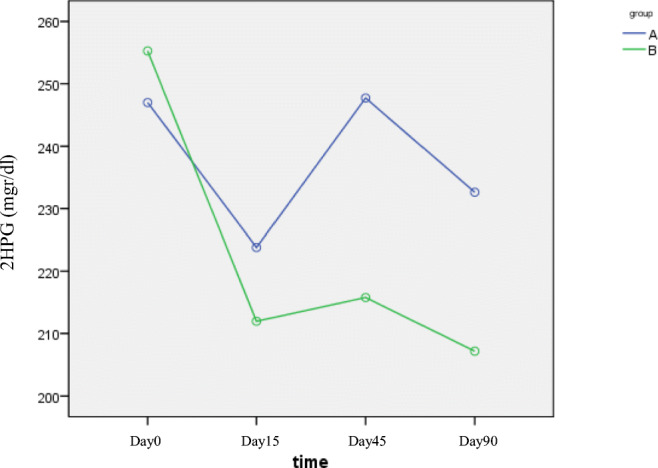
postprandial glucose changes during different time intervals (A: B. integerrima, B: Metformin). Postprandial glucose that significantly reduced at the end of the study in both groups. (P = 0.01, P = 0.018).
The initial HbA1c in Group A and B was 8.41 ± 1.73% and 7.83 ± 1.56% respectively that there was no significant difference between the groups (P = 0.13).
Mean HbA1c at the end of the study was 7.59 ± 1.61% in group A and 7.22 ± 1.3% in group B with no significant difference between the two groups (P = 0.34).
But in both groups, HbA1c, comparing to prior to the study was significantly reduced. (In Group A: P = 0.005, and the group B: P = 0.002, Fig. 5).
Fig. 5.
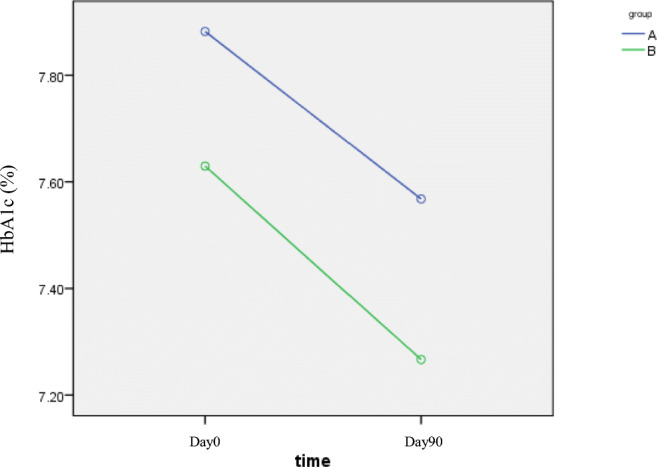
HbA1c changes during different time intervals (A: B. integerrima, B: Metformin). There was no difference between HbA1c reduction between the two groups (P = 0.982)
The FPG levels were not differ in the four periods (P for trend = 0.8).
The 2HPG levels were not differ in the four periods (P for trend = 0.81).
The HbA1c levels were not differ in the four periods (P for trend = 0.70).
HbA1c differences, pre- and post treatment, in each group were calculated; also no significant difference between the two groups was observed (P = 0.89, Table 2).
Table 2.
Comparison of the mean of FPG, 2HPG and HbA1c in both groups at different times (A: B. integerrima,B: Metformin)
| Group A | Group B | *P for trend | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| FPG Mg/dl | |||
| Day0 | 182 ± 51 | 167 ± 50 | 0.80 |
| Day15 | 189 ± 69 | 153 ± 48 | |
| Day45 | 169 ± 57 | 156 ± 61 | |
| Day90 | 166 ± 60 | 148 ± 48 | |
| 2HPG Mg/dl | |||
| Day0 | 274 ± 103 | 261 ± 104 | 0.81 |
| Day15 | 264 ± 121 | 221 ± 82 | |
| Day45 | 253 ± 104 | 226 ± 89 | |
| Day90 | 232 ± 95 | 207 ± 77 | |
| HbA1c percent | |||
| Day0 | 8.4 ± 1.7 | 7.8 ± 1.5 | 0.7 |
| Day90 | 7.5 ± 1.6 | 7.2 ± 1.3 | |
*P for trend was obtained from Generalized Linear Model models
Fructosamine, between two groups did not arrive at a statistical significance (286 ± 66 μmol/L vs. 281 ± 91 μmol/L, p = 0.05).
The variation of AST and ALT did not reach to a difference (P = 0.92 and P = 0.96). But a slight increase in AST 1 and 2 on 14th and 45th was observed in group A (P = 0.01 and P = 0.026).Significant decrease in triglycerides was observed in the first month in group A (P = 0.02). But ultimately, the process of reducing triglycerides were similar in both groups (P = 0.74).However, no significant difference was seen in cholesterol level between two groups, but it tends to decrease from 187 to 169 in group A, while remained at 186 in group B. Although, a borderline significant risk reduction was found in cholesterol level; with no specific time pattern (P = 0.008).
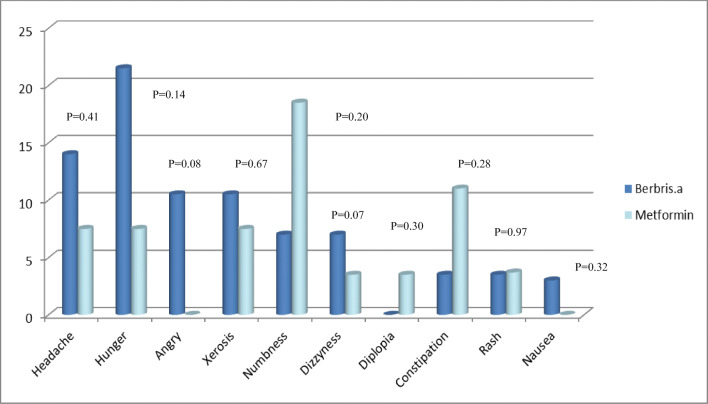
HDL1 appears to reduce from 43 to 39 in Group A at time 2 (45th day); a significant decrease (P = 0.006). yet, at the end of the study, no significant differences were observed between two groups (P =0.32). Frequency of complications in group A was as follows: hunger in 6 patients, headache in 4 patients, the most common complaint, anger and xerostomia in 3, numbness and dizziness in 2, blurred vision, constipation, rash and nausea in 1. Still, these effects were not statistically different between two groups (Fig. 6).N0 cases of double vision, abnormal behavior, confusion and ataxia, abdominal pain, anorexia, diarrhea, urticaria, sore throat, seizure and melena were reported during the study. In group A, 76% and 23% of patients in group B complained of sweating. This was significantly higher in group A (P = 0.02).But at the end of the study, after treatment, there was no complaints of sweating in group A patients (group A, p = 0.001 and group B, p = 0.32); suggesting the therapeutic effect of B. integerrima on autonomic nervous system.In the entire study, no cases of hepatosplenomegaly, lymphadenopathy and jaundice were observed in each of the groups.
Fig. 6.
Comparison of complications in the two groups at the end of the study
Discussion
The present study, to evaluate the safety & efficacy of B. integerrima root extract in patients with type 2 diabetes compared to metformin, showed similar effect of B. integerrima in reducing FPG, 2hPG, HbA1c and fructosamine. It also caused weight loss in patients, the same as metformin. None of these drugs had effect on blood pressure. Also no significant side effect was found. In the animal studies, the therapeutic effects of B. aristata root extract on blood glucose [11, 17–27], cholesterol [21, 28–30], liver enzymes [21, 31–35] have been demonstrated. It should be noted that at the beginning of our study 5 patients were excluded in B. integerrima group due to poor glycemic control and received insulin treatment. All these patients were assessed with FPG > 200 and PPG > 300 at the time of inclusion and had received hypoglycemic medication before entering. Accordingly, these patients were not appropriate candidates for entry and possibly discontinuing the medication increased their blood glucose. Also, it indicates that the therapeutic dose of the drug did not have enough power and potency in elevated blood sugar and sharp decline in insulin level. In this study, FPG in the group treated with metformin was reduced more quickly in the first two weeks. Postprandial glucose also declined in both groups and no significant differences were observed between two groups, but the greatest effect of B. integerrima capsules was seen in the first month. Measurement of fructosamine, conducted after two weeks, showed no significant difference between the two groups, although it was lower in patients treated with B. integerrima capsules. A significant decrease in HbA1c was observed after 3 months; there was no difference in the two groups. Despite the lack of information on the side effects of the drug in humans, our study was carried out with a minimum dose, but our results were consistent with other studies in animals. [11, 18–24]
Data analysis was carried out for all existing patient in each stage (day 0–15-45 and 90). In a study conducted in 2007 [21], the effect of B. aristata stem bark on weight loss in diabetic rats was shown that the same result was obtained in our study with no difference with the groups taking metformin. The hepatotoxicity effect of B. aristata was evaluated in a study in 2013.Histological assessment of the liver indicated reduction in destruction of hepatic cords and reduction of necrosis and inflammation. Also, ethanolic extract of B. aristata caused significant hepatoprotective activity against carbon tetrachloride induced hepatotoxicity in rats. Furthermore, in other studies on laboratory animals, hepatoprotective and therapeutic activity of B. aristata on lowering liver enzymes have been shown [21, 31]. In our study, one of the patients experienced an increase in AST enzyme level, 3 times the normal (118 IU/mL) at the baseline that was excluded from the study. But, in the next follow-ups, still raised level was determined (105 IU/mL, 120 IU/mL) and fibroscan detected fatty liver grade 3.This means that probably, the elevated AST level was not any of the side effects of medication, because after withdrawal the tests remained the same; likely due to fatty liver disease.
But, in other patients, no significant impairment of liver function tests as: AST, ALT, Alkaline phosphatase, Bilirubin, PT, PTT was observed and there was no difference with the control group. Regarding the reduction of liver enzyme, our study is inconsistent with the results of animal studies. This could be due to the low dose of drug compared to the animal studies. In two studies conducted in 2007 and 2011 on laboratory animals significant reduction in lipid profile (Triglyceride, Cholestrol, LDL) was demonstrated [21, 28]. In other trials performed in 2013 and 2015 the effect of B. aristata combined with Silybum marianum on lipid profile was examined and significant reduction in lipid profile and increased insulin secretion was observed [29, 30]. A significant decrease in triglycerides and cholesterol levels was seen in our study, in the first month, compared to the control group, but there was no significant difference at the end of the study. Failure to reduce lipids and liver tests in our study suggests that probably, higher therapeutic doses are required to impact on liver enzymes and lipid changes.
No significant changes were observed in WBC, Hemoglobin, Platelet, Creatinin, Urea, and Urine analysis as compared to the patient’s arrival. No complication associated with side effects leading to patients exclusion was seen. In general, most patients complained of weakness, hunger and headache, and symptoms such as numbness or tingling in hands and feet, nervousness, dry mouth, blurred vision, constipation, rash and nausea was developed in a few patients which improved by medical treatment. On the whole, there was no significant difference in complications between the two groups. At baseline, 16 patients (76%) complained of sweating; however it improved at the end of the study. This means that this drug may have excellent therapeutic effect in reducing sweating and improving the autonomic nervous system in diabetes that requires further investigation in this area.
Limitations:
Firstly, because the safety of the drug in humans was examined for the first time in our study, we had dose limitation. A low dose (480 mg daily) was considered which led to exclusion of 5 patients due to lack of glycemic control.
It appears that this low dose alone cannot control blood glucose in severe cases and more studies with higher doses of this drug are recommended.
The second limitation was the small sample size due to lack of information on adverse effects of drug.
The third limitation was the exclusion of patients from the study for different reasons, which were replaced during the study and the final analysis was performed on patients who had finished the project.
AS DPP-4 inhibitor mechanism of the drug was not identified at the time of study design, we compared B. integerrima root with metformin. Further studies of higher dose of drug with larger sample size in comparison with class of Alpha-glucosidase inhibitors and DPP-4 inhibitors as well as the use of the drug in the form of a combination regimen and also, studies to isolate active phytoconstituents of this drug are recommended.
Conclusion
This study showed that short time (12 weeks) ingestion of B. integerrima roots extract not only reduces blood glucose in type 2 diabetic patients equally to metformin (efficacy), but also did not cause any particular medical complications (safety).It also lowered cholesterol and weight of patients. Due to the positive and significant effects of B. integerrima root on blood glucose in diabetic patients and safety of this drug, it might be used in the treatment of type 2 diabetes in drug-naive patients as well as, in mild and early stage cases as an effective and safe drug choice.
Acknowledgements
This study is a part of the collaborative project supported by vice chancellor for research, Physiology Research Center of Kerman University of Medical Sciences and the pharmaceutical company (Exir) as well as internal medicine resident thesis. The authors would like to appreciate all patients participating in the project as well as the pharmaceutical company (Exir) that prepared metformin and placebo and Dr. Fariba Sharififar for supervising the stages of extraction.
Abbreviations
- (FPG)
Fasting plasma glucose
- (2HPG)
2 h post prandial glucose
- (cr)
creatinine
- (TG)
Triglycerides
- (chol)
Cholestrol
- (HDL)
High density Lipoprotein
- (AST)
Aspartate aminotransferase
- (ALT)
Alanine aminotransferase
- (Alk)
Alkaline phosphatas
- (PTT)
partial thromboplastin time
- (PT)
prothrombin Time
- (bil)
bilirubin
- (CBC)
complete blood count
- (U/A)
urine analysis
Authors’ contribution
Mojgan. Sanjari designed the experiment and prepared the initial proposal in collaboration with the Fatemeh Mir Rashidi. Behrang Shamsi nejad implemented the project and collected the data. Payam Khazaeli prepared the extract in the School of Pharmacy. Zohreh Safi performed sampling and patients follow up. All authors have collaborated in writing the paper.
Compliance with ethical standards
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mojgan Sanjari, Email: mjnsanjari@gmail.com.
Behrang Shamsinejad, Email: msanjari@kmu.ac.ir.
Payam Khazaeli, Email: pkhazaeli@kmu.ac.ir.
Zohreh Safi, Email: kerman.physiology@gmail.com.
Fatemeh Mirrashidi, Email: fatemeh.mirrashidi@gmail.com.
Ahmad Naghibzadeh-Tahami, Email: anaghibzadeh61@gmail.com.
References
- 1.Najafipour H, Sanjari M, Shokoohi M, Haghdoost AA, Afshari M, Shadkam M, et al. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes and its predictors in general population aged 15 to 75 years: a community-based study (KERCADRS) in southeastern Iran. J Diabetes. 2015;7(5):613–621. doi: 10.1111/1753-0407.12195. [DOI] [PubMed] [Google Scholar]
- 2.Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison's principles of internal medicine: McGraw-Hill Professional Publishing; 2018. p. 2859–868.
- 3.Patel D, Kumar R, Laloo D, Hemalatha S. Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac J Trop Biomed. 2012;2(5):411–420. doi: 10.1016/S2221-1691(12)60067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarnishrinivas K, Dhira A. Possible involvment of L-argenune-nitric oxide(NO)-cyclic guanosine monophosphate (c-GMP)signaling pathway in the antidepressant activity of berbeine choloride. Eur J Pharmacol. 2007;13(1–2):77–83. doi: 10.1016/j.ejphar.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Padmaja V, et al. Antidiarrheal activity ,chemical and toxicity profile of Berberis integerrima. Pharm Biol. 2011;49(1):94–100. [DOI] [PubMed]
- 6.Block W. Berberine is superior to metformin. Life Enhancement 2011. http://www.life-enhancement.com/magazine/article/2439-berberine-is-superior-to-metformin
- 7.Al-masri IM, M.K. Mohammad, Tahaa MO. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J Enzyme Inhib Med Chem 2009;24(5):1061–1066. [DOI] [PubMed]
- 8.Lazavi F, Mirmiran P, Sohrab G, Nikpayam O, Angoorani P, Hedayati M. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: a randomized clinical trial. Complement Ther Clin Pract. 2018;31:170–174. doi: 10.1016/j.ctcp.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Fogacci F, Grassi D, Rizzo M, Cicero AF. Metabolic effect of berberine–silymarin association: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Phytother Res. 2019;33(4):862–870. doi: 10.1002/ptr.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komal S, Ranjan B, Neelan C, Birendra S, Kumar SN. Berberis aristata:Areview. Int J Res Ayurveda Pharm. 2011;2(2):383–8.
- 11.Semwal BC, Gupta J, Singh S, Kumar Y, Giri M. Antihyperglycemic activity of root of Berberis aristata D.C. in alloxan-induced diabetic rats. Int J Green Pharm. 2009;3:259–262. doi: 10.4103/0973-8258.56288. [DOI] [Google Scholar]
- 12.Sood H, Kumar Y, Gupta VK, Arora DS. Scientific validation of the antimicrobial and antiproliferative potential of Berberis aristata DC root bark, its phytoconstituents and their biosafety. AMB Express. 2019;9(143). [DOI] [PMC free article] [PubMed]
- 13.association Ad Diagnosis and classificationof diabetes mellitus. Diabet Care Journal. 2011;34(8):15–16. [Google Scholar]
- 14.Upwar N, Patel R, Waseem N, Mahobia NK. Hypoglycemic effect of methanolic extract of Berberis aristata DC stem on normal and streptozotocin induced diabetic rats. Int J Pharm Pharm Sci. 2011;3(1):222–224. [Google Scholar]
- 15.Schulz K, Altman D, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 16.M V, S.V a. The Efficacy and Complications of Metformin Produced by Iranian Co in Comparison with Canadian Co. J Adv Med Biomed Res. 2010;18(73):1–10.
- 17.Gupta JK, Mishra P, Aap R, Mazumder M. Blood glucose lowering potential of stem bark of Berberis aristata Dc in Alloxan- induced Diabetic rats. Iranian Journal of Pharmacology & Therapeutics. 2010;9(1):21–24. doi: 10.1111/j.1365-2710.1984.tb00915.x. [DOI] [Google Scholar]
- 18.Anil P, Manish S. Antidibetic activity of extract of berberis aristata root in streptozotocin induced diabetic rats. Pharmacologyonline. 2010;2:179–185. [Google Scholar]
- 19.Singh J, Kakkar P. Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J Ethnopharmacol. 2009;123(1):22–26. doi: 10.1016/j.jep.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Sharma k, al. e. berberis aristata article review ijrap. 2011;12(2):383–388. [Google Scholar]
- 21.B S, K S, N C, R B, K D. Anti-diabetic activity of stem bark of Berberis aristata D.C. in alloxan induced diabetic rats. Int. J. Pharmacol. 2007;6(1).
- 22.Francesco Di P, Pietro P, Nicola V, Luca M, Simona M, Giulio M. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin Pharm. 2013;5:167–174. doi: 10.2147/CPAA.S54308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitinkumar U, Roshan P, Naheed W, Naveenkunar M. Hypoglycemic effect of methanolic extract of berberis aristata dc stem on normal and streptozotocin induced diabetic rats. Int J Pharm Pharm Sci. 2011;3(1):222–4.
- 24.Manjari M, Vijay J, Anita S. Phytochemical, Antidiabetic, and Cytoprotective properties of Berberis aristata DC. Root Extracts Pharmaceutical Crops. 2012;3:64–68. doi: 10.2174/2210290601203010064. [DOI] [Google Scholar]
- 25.Akhtar MS, Sajid MS, Ahmad M. Hypoglycemic effect of Berberis aristata root, its aqueous and methanolic extract induced diabetic rabbits. Pharmacol Online. 2008;2:845–856. [Google Scholar]
- 26.Ahmad R, Prakash SS, Maurya R, Rajendran S, Arya K, Srivastava Arvind K. Mild Antihyperglycemic activity in Eclipta alba, Berberis aristata, Betula utilis, Cedrus deodara, Myristica fragrans and Terminalia chebula. Ind J of Sc ience and Technology. 2008;5(1):1–6. [Google Scholar]
- 27.Shah K, Chauhan NS, Semwal BC, Rohit B, Kalyani D. Antidiabetic activity of stem bark of Berberis aristata D.C. (Berberdiaceae) in alloxan induced diabetic rats. Int J Pharm. 2010;1(6).
- 28.Faiza Abdul R, Rafeeq Alam K, Zeeshan F, Syeda A. Effect of Berberis aristata on lipid profile and coagulation parameters. Afr J Pharm Pharmacol. 2011;7(5):943–947. [Google Scholar]
- 29.Derosa G, Romano D, D'Angelo A, Maffioli P. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis. 2015;1(239):87–92. doi: 10.1016/j.atherosclerosis.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 30.Derosa G, Bonaventura A, Bianchi L, Romano D, D'Angelo A, Fogari E, et al. Berberis aristata/Silybum marianum fixed combination on lipid profile and insulin secretion in dyslipidemic patients. Expert Opin Biol Ther. 2013;11(13):1495–1506. doi: 10.1517/14712598.2013.832751. [DOI] [PubMed] [Google Scholar]
- 31.Navdeep D, Rani W, Verma RB, Pinky P. Hepatoprotective activity of berberis aristata root extract against chemical induced acute hepatotoxicity in rats. Asian J Pharm Clin Res. 2013;6:53–56. [Google Scholar]
- 32.Unkeshwar P, Nasiruddin M, Fayazuddin M, Khan RA, Khan AA. Tajuddin. Evaluation of hepatoprotective activity of berberis aristata against carbon tetrachloride induced hepatotoxicity in rats. Int J Pharm Pharm Sci. 2013;5:107–10.
- 33.Brijesh K, Tiwari KR. Evalution of the Hepatoprotective and antioxidant effect of Berberis asiatica against exeperimentally induced liver injury in rats. Int J Pharm Pharm Sci. 2010;1(2).
- 34.Gilani A, Janbaz K. Preventive and curative effects of berberis aristata fruit extract on paracetamol - and CCl4-induced hepatotoxicity. Phytother Res. 1995;9(7):489–494. doi: 10.1002/ptr.2650090705. [DOI] [Google Scholar]
- 35.Janbaz K, Gilani A. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71(1):25–33. doi: 10.1016/S0367-326X(99)00098-2. [DOI] [PubMed] [Google Scholar]