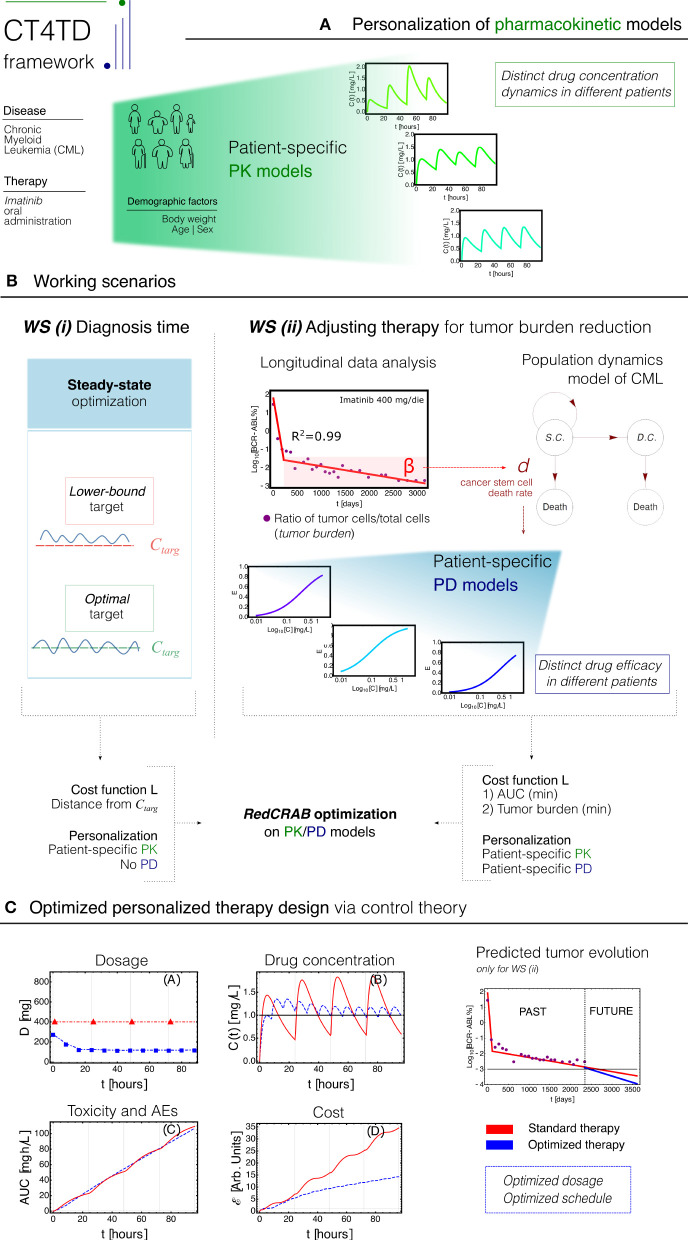
Figure 1.
CT4TD pipeline. (A) The CT4TD framework employs demographic factors such as body weight, age, and sex to define patient-specific parameters of the pharmacokinetic models. We here focus on the case of Imatinib administration in Chronic Myeloid leukemia (CML). (B) CT4TD manages two working scenarios: (i) at time of diagnosis, CT4TD can be used to reach given optimal/lower-bound drug concentration targets, e.g., from clinical studies (steady state optimization); (ii) when longitudinal data on tumor burden variation under standard therapy are available, CT4TD fits the data points with a hierarchical population dynamics model of CML, and this allows to estimate patient-specific pharmacodynamics (PD) parameters, based on the observed cancer cell death rate. In both scenarios, optimization on pharmacokinetics/pharmacodynamics (PK/PD) models is performed via RedCRAB, on distinct cost functions, aimed at: either being close to given target concentrations (and strictly larger in the lower-bound case)—WS (i); minimizing the Area Under the Curve (AUC) and the tumor burden—Working Scenario (WS) (ii). (C) optimized personalized dosage and schedule are returned, allowing to measure in silico the differences with respect to standard administration, in terms of dosage, drug concentration, cost, and AUC. WS (ii) allows to predict the tumor burden evolution in case of an optimized therapy.