Abstract
Skeletal muscle has remarkable regenerative abilities regulated by a highly orchestrated process involving the activation of cellular and molecular responses, which are dependent on satellite cells. These cells maintain the stem cell population and provide numerous myogenic cells that proliferate, differentiate, fuse and lead to new myofiber formation for a functional contractile tissue. We have isolated and characterized satellite cells obtained from human biopsies and established an in vitro model of myogenesis, evaluating muscle regeneration, monitoring the dynamic increases of the specific myogenic regulatory factors and the final formation of multinucleated myofibers. As the skeletal muscle is an endocrine tissue able of producing many substances that can act on distant organs, and it can be physiologically modulated by a variety of hormones, we embarked in a project of characterization of muscle cell endocrinology machinery. The expression of a large array of hormone receptors was quantified during the process of myogenesis. The results obtained showed a significant and generalized increase of all the tested hormone receptors along the process of differentiation of human cultured cells from myoblasts to myocytes. Interestingly, also the production of the myokine irisin increased in a parallel manner. These findings point to the human cultured myoblasts as an ideal model to characterize the skeletal muscle endocrine machinery and its hormonal regulation.
Keywords: Skeletal muscle, Satellite cells, Myogenesis, Skeletal muscle endocrinology, Hormone receptors
Introduction
Skeletal muscle is the most abundant tissue in the human body, accounting for about 40–45% of total body weight. It plays an important role in controlling physical activity, including voluntary locomotion, postural behavior, and breathing. Moreover, it has an extraordinary ability to adapt to physiological demands, such as growth, and to regenerate new muscle fibers after damage by injury or intense physical activity [1].
The regeneration and remodeling of skeletal muscles are extremely complex biological processes, in which skeletal muscle stem cells (also known as satellite cells, SCs) are involved. The SCs are located under the basal lamina of the myofiber; this position, between the myofiber and the surrounding extracellular matrix (ECM), is the reason Alexander Mauro gave them this name in 1961 [2]. In healthy adult mammalian muscle, SCs are predominantly quiescent (phase G0) and represent 2.5–6% of all nuclei of a given fiber; however, after injury or degeneration, SCs become activated and can generate large numbers of new myofibers within just few days [3]. Like stem cells, satellite cells also self-renew to maintain their own population, re-establishing their numbers and quiescent state by homing back to highly specialized niches, thus allowing future rounds of regeneration [4]. Specific temporal factors, called Myogenic Regulatory Factors (MRFs), members of the basic helix-loop-helix (bHLH) family of transcription factors including Myf-5, MyoD1, Myogenin and MRF4, are an essential group of four muscle-specific proteins responsible for acting at multiple points in the muscle lineage to cooperatively establish the skeletal muscle phenotype [4, 5].
Besides its well-known structural and biomechanical functions for the purpose of movement, the skeletal muscle is considered a secretory organ, capable of producing several substances called myokines, which can act on the muscle itself, on nearby tissues, and on distant organs, in an autocrine, paracrine and endocrine fashion, respectively [6–8]. These functions can be physiologically modulated by physical stimuli and by cytokines, mineral ions and hormones, or can be modified by sarcopenia, morphological modification of muscle fibers and/or endocrinopathies.
The evaluation of the skeletal muscle as a secretory organ is not fully understood, neither it is the endocrine control of muscle function and differentiation [9]. A future chapter in the endocrine discipline will certainly be musclecrinology. The aim of our study was to evaluate the endocrine machinery in an in vitro cellular model of myogenesis obtained from human skeletal muscle biopsies. The understanding of hormonal production and regulation in the skeletal muscle remodeling may contribute to the identification of new possible therapeutic targets in pathologies in which the myogenesis and/or the function of mature myocytes is affected.
Materials and Methods
Isolation of Human Skeletal Muscle-Derived Cells (hSkMCs)
Primary cultures were isolated from human skeletal muscle biopsies of 3 healthy adult volunteers undergoing plastic surgery, after signing an informed consent in accordance with a protocol approved by the Local Ethics Committee of AOU Careggi, Firenze (Italy), for human studies (Rif. N. 14.017), as well as the ethical standards stated in the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. The minced specimens were processed within 3 h from the operation and enzymatically digested for 3 h at 37 °C in Ham’s F12 Coon’s modification medium (Sigma-Aldrich) supplemented with 20% fetal bovine serum (FBS) and 3 mg/ml collagenase type I (C-0130, Sigma-Aldrich). The tissues were then mechanically dispersed by pipetting and passed through a sterile 100 μm stainless steel tissue sieve to remove any large debris. The undigested tissue trapped in the sieve was discarded, while the infranatant containing the hSkMC fraction was collected and the cells sedimented by centrifugation at 300×g for 5 min. The cells were then pre-plated into 100 mm Petri dishes for 1 h at 37 °C to remove fibroblasts which adhere to plastic more avidly than satellite cells. Afterwards, the resulting suspension was seeded in 100 mm tissue culture plates at 37 °C in humidified atmosphere with 5% CO2 using a skeletal muscle cell growth medium (GM) composed of Skeletal Muscle Cell Basal Medium (PromoCell GmbH, cod. C-23260) supplemented with 5% Fetal Calf Serum (FCS), 50 µg/ml fetuin, 10 ng/ml Epidermal Growth Factor (EGF), 1 ng/ml basic Fibroblast Growth Factor (bFGF), 10 µg/ml insulin, 0.4 µg/ml dexamethasone and 100 IU/ml penicillin, 100 μg/ml streptomycin (Table 1). The medium was refreshed twice a week and the cells were used for further subculturing or cryopreservation upon reaching 5 × 103 cells/cm2. Cells at the early passages (from 1 to 4) were used for all the experiments. All tissue culture plates utilized for cell amplification and experiments were previously coated with Matrigel® (BD Biosciences) in order to increase cell attachment, proliferation and to maintain cell phenotype [10].
Table 1.
Supplements and concentrations after their addition to the basal medium for growth medium and myogenic differentiation medium used in the experiments, respectively
| Growth medium | Myogenic differentiation medium | |
|---|---|---|
| Fetal calf serum | 0.05 ml/ml | – |
| Fetuin | 50 µg/ml | – |
| Epidermal growth factor | 10 ng/ml | – |
| Basic fibroblast growth factor | 1 ng/ml | – |
| Insulin | 10 µg/ml | 10 µg/ml |
| Dexamethasone | 0.4 µg/ml | – |
hSkMC Characterization and Multipotency Evaluation
The characterization of the hSkMC cell lines was performed analyzing the presence of the surface markers of mesenchymal stem cells and specific markers of the isolated satellite cells by flow cytometry analysis and by studying their multipotency toward the myogenic, the adipogenic and the osteogenic phenotypes, as previously described [11].
Flow Cytometry
hSkMC lines were evaluated by flow cytometry with a CyFlow®Space cytometer (Sysmex Partec), equipped with FlowMax® software. The antibodies used (Abcam) were directed against the following antigens (the tags are given in parentheses): CD44 (PE/Cy7), CD90 (APC), CD105 (FITC), CD45 (PerPC), CD34 (PE) and CD56 (PerCP Cy5.5) and PAX-7 (FITC). Each antibody was diluted according to manufacturer's instruction. Briefly, 1 × 105 cells were labeled with antibodies in PBS with 1% bovine serum albumin (BSA) for 20 min RT in the dark, then washed once and promptly analyzed.
Myogenic Differentiation
hSkMC lines at 70% of confluence were cultured with a specific myogenic medium (MM): Skeletal Muscle Cell Basal Medium (PromoCell GmbH, cod. C 23260) supplemented with 10 µg/ml insulin, 100 IU/ml penicillin and 100 μg/ml streptomycin. The medium was refreshed twice a week. The expression of the myogenic phenotype was evaluated by microscopic observations of the multinucleated cells formation, by immunofluorescence of the myosin heavy chain (MHC) and by gene expression analysis, after 10 days of induction (Table 1).
Adipogenic Differentiation
hSkMC lines were cultured with a specific adipogenic medium (AM): Ham’s F12 Coon’s modification medium supplemented with 10% (FBS), 100 IU/ml penicillin, 100 μg/ml streptomycin and 1 μM dexamethasone, 1 μM bovine insulin, 0.5 mM isobutylmethylxanthine (IBMX). The medium was refreshed twice a week. The expression of the adipogenic phenotype was evaluated on cells cultured in AM for 10 days by cytochemical staining with Oil Red O and brightfield observations (Axiovert 200, Zeiss).
Osteogenic Differentiation
hSkMCs were plated on tissue culture dishes at a cell density of 1 × 104 cells/cm2 and grown to 70–80% confluence. Afterwards, the medium was switched to osteogenic medium (OM): Ham’s F12 Coon’s modification medium supplemented with 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 nM dexamethasone, 0.2 mM sodium l-ascorbyl-2-phosphate, and 10 mM β-glycerol phosphate. The medium was refreshed twice a week. The expression of the osteoblastic phenotype was evaluated at 20 days from induction by monitoring the production of mineralized nodules by cytochemical staining. The cells were washed with DPBS (two times), fixed in 4% paraformaldehyde (PFA)/DPBS for 15 min, and washed with ultrapure water (three times). Calcium mineral deposits were stained with 1 µg/ml calcein added to the OM and nuclei were counterstained with 1 µg/ml bisbenzimide for 5 min; calcium mineralized deposits were stained in fluorescent green, nuclei in blue, and then visualized in epifluorescence microscopy (Axiovert 200, Zeiss).
Immunofluorescence
hSkMCs were seeded into 24-well plates (1 × 104 cells/well) and cultured for 24 h in GM. Afterwards, cells were fixed for 10 min with 4% paraformaldehyde and permeabilized for 10 min with 0.2% Triton X 100 at RT. Cells were treated for another 30 min at 37 °C with RNAse in 2% bovine serum albumin (BSA) in order to degrade RNA and block non-specific sites. Samples were then incubated overnight with primary antibody for PAX-7 and MHC (Abcam, Cambridge, UK) in PBS at 4 °C. After extensive washes with PBS, goat anti-mouse IgG (H + L) SuperClonal secondary antibody, Alexa Fluor 488 conjugate (Thermo Fischer Scientific, Waltham, MA, USA) was incubated for 1 h at room temperature in the dark. Subsequently, nuclei were counterstained with 10−5 M propidium iodide. Samples were then washed with PBS for observation in laser scanning confocal microscopy (LSCM), using an LSM 510 Meta microscope (ZEISS, Oberkochen, Germany) [12].
RNA Extraction and Real-Time qPCR Analysis
Gene expression analysis in the hSkMCs was performed in GM and after 9 days of induction in MM. The genes included in the analysis were PAX-7, MyoD-1, Myf-5, MRF-4, Myogenin, Desmin, MHC, Irisin, and specific hormone receptor genes (VDR, TRα, TRβ, GCR, IGF-1, PTH1R, LRP-5, LRP-6). Target gene expression was normalized to 40S ribosomal protein S18 (RPS18). All procedures for amplification were previously described [13] (Table 2).
Table 2.
Primers and TaqMan probes used for the experiments
| Gene | Primer sequences (5′–3′) and TaqMan probes | Amplicon size (bp) | Tm (°C) |
|---|---|---|---|
| PAX-7 for | GGTACCGAGAATGATGCGG | 124 | 55 |
| PAX-7 rev | CCCATTGATGAAGACCCCTC | ||
| Pax-7 Probe | 6-FAM/AGCTGATTG /Zen/ACCCGGCCTTGG/3IABkFQ | ||
| MyoD-1 for | GACGTGCCTTCTGAGTCG | 148 | 55 |
| MyoD-1 rev | CTCAGAGCACCTGGTATATCG | ||
| MyoD-1 Probe | 6-FAM/CGCTGCTCT/Zen/CTCCCTCGCTG/3IABkFQ | ||
| Myf-5 for | ATGCCATCCGCTACATCG | 145 | 55 |
| Myf-5 rev | ACAGGACTGTTACATTCGGC | ||
| Myf-5 Probe | 6-FAM/CCCCACCTC/Zen/CAACTGCTCTGAT/3IABkFQ | ||
| MRF-4 for | CCCTGGAATGATCGGAAACA | 95 | 55 |
| MRF-4 rev | CTTCAGCTACAGACCCAAACA | ||
| MRF-4 Probe | 6-FAM/ATCTTGAGG/ZEN/GTGCGGATTTCCTGC/3IABkFQ | ||
| Myogenin for | AGCGAATGCAGCTCTCAC | 150 | 55 |
| Myogenin rev | TGTGATGCTGTCCACGATG | ||
| Myogenin Probe | 6-FAM/TGACCCTAC/Zen/AGATGCCCACAACC/3IABkFQ | ||
| MHC for | GAGTCCTTTGTGAAAGCAACAG | 143 | 55 |
| MHC rev | GCCATGTCCTCGATCTTGTC | ||
| MHC Probe | 6-FAM/CAAGTCTTC/Zen/CCCATGAACCCTCCC/3IABkFQ | ||
| Desmin for | AACGCGATCTCCTCGTTG | 101 | 55 |
| Desmin rev | GAGAACAATTTGGCTGCCTTC | ||
| Desmin Probe | 6-FAM/CAATTCTGC/ZEN/GCTCCAGGTCAATGC/3IABkFQ | ||
| VDR for | CCGCATCACCAAGGACAA | 112 | 62 |
| VDR rev | CTTCCTCTGCACTTCCTCATC | ||
| VDR Probe | 6-FAM/TGTGGACAT/ZEN/CGGCATGATGAAGGA/3IABkFQ | ||
| TRα for | TCCCTAGTTACCTGGACAAAGA | 133 | 59 |
| TRα rev | GGATGGAGGTTCTTCTGGATTG | ||
| TRα Probe | 6-FAM/ACAGCGGTA/ZEN/GTGATAACCAGTTGCC/3IABkFQ | ||
| TRβ for | CTTCCAAACGGAGGAGAAGAA | 115 | 59 |
| TRβ rev | CGTGATACAGCGGTAGTGATAC | ||
| TRβ Probe | 6-FAM/TGTGTAGTG/ZEN/TGTGGTGACAAAGCCA/3IABkFQ | ||
| GCR for | TGGTCCTGTTGTTGCTGTT | 103 | 55 |
| GCR rev | CTTCCCTGGTCGAACAGTTT | ||
| GCR Probe | 6-FAM/TAAGCTCTC/ZEN/CTCCATCCAGCTCCT/3IABkFQ | ||
| IGF-1 for | CAGCAAGTGAGGAGAGGAAC | 131 | 59 |
| IGF-1 rev | GTGTGAGAAGACCACCATCAA | ||
| IGF-1 Probe | 6-FAM/TCGAAGAGA/ZEN/GCAAATGCACATCCCT/3IABkFQ | ||
| LRP-5 for | CCCAGTCTGTCCAGTACATG | 134 | 59 |
| LRP-5 rev | CTCAGAGACCAACCGCATC | ||
| LRP-5 Probe | 6-FAM/CCAACCTCA/ZEN/ATG/3IABkFQ | ||
| LRP-6 for | CCCATTTGTGTTTGATGTCTCC | 137 | 60 |
| LRP-6 rev | CAAGTCTGTCCTTCGAGCTAAA | ||
| LRP-6 Probe | 6-FAM/AAACCTGCA/ZEN/AAGATGGTGCCACAG/3IABkFQ | ||
| PTH-1R for | GGGAAGCCCAGGAAAGATAAG | 125 | 58 |
| PTH-1R rev | CACAGGATGTGGTCCCATT | ||
| PTH-1R Probe | 6-FAM/TGCCTCCTT/ZEN/GTCCTCCTCAGACTC/3IABkFQ | ||
| Irisin for | ACTATGTACTCCGTATCCTCCTC | 126 | 55 |
| Irisin Rev | TGTCATCGGATTTGCCATCT | ||
| Irisin Probe | 6-FAM/CCAGCAGAA/ZEN/GAAGGATGTGTCGGAT/3IABkFQ | ||
| RPS18 for | GATGGCAAAGGCTATTTTCCG | 132 | 60 |
| RPS18 rev | TCTTCCACAGGAGGCCTAC | ||
| RPS18 Probe | 6-FAM/TTCAGGGAT(ZEN/CACTAGAGACATGGCTGC/3IABkFQ |
TaqMan probes with F as reporter fluorochrome (6-carboxyfluorescein [6-FAM]) and ZEN as quencher. Fluorochrome (Iowa Black FQ); bp base pairs of amplicon size; Tm melting temperature (°C)
Statistical Analysis
All gene expression analyses were performed in tetraplicate and each experiment was repeated three times. All data were expressed as means ± SD and are the number of mRNA molecules of the specific genes normalized to the housekeeping RPS18 mRNA. Statistical differences among mean values were analyzed using ANOVA and a post hoc sequentially rejective multiple Bonferroni test, with predetermined (default) experimentwise probability αT = 0.05, comparing two groups: the value of the specific gene after 9 days of myogenic induction with respect to the control in proliferating medium.
Results
Isolation and Characterization of hSkMCs
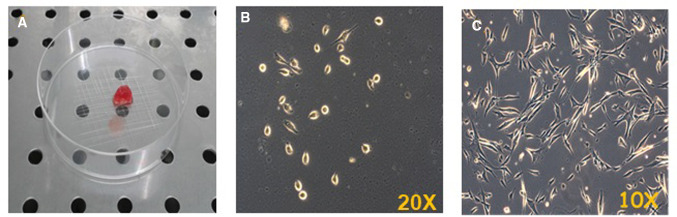
The cell populations derived from human skeletal muscle biopsies (Fig. 1a), obtained by surgical resection, were amplified in Matrigel® coated plates in order to increase cell adherence and maintain cell phenotype. In fact, the isolated cells, when plated for the first time in a dish (passage 0), attach showing a rounded shape that persists for 2–3 days, with a slow proliferation rate (Fig. 1b). After that period, cells become flatter and show an elongated morphology with 2–4 cytoplasmic extensions (Fig. 1c).
Fig. 1.
Photo of human skeletal muscle biopsy (a); representative observations of primary culture of hSkMCs in phase contrast microscopy at passage 0 after 1 day in the dish; objective ×20 (b) and after 3 days in the dish; objective ×10s (c)
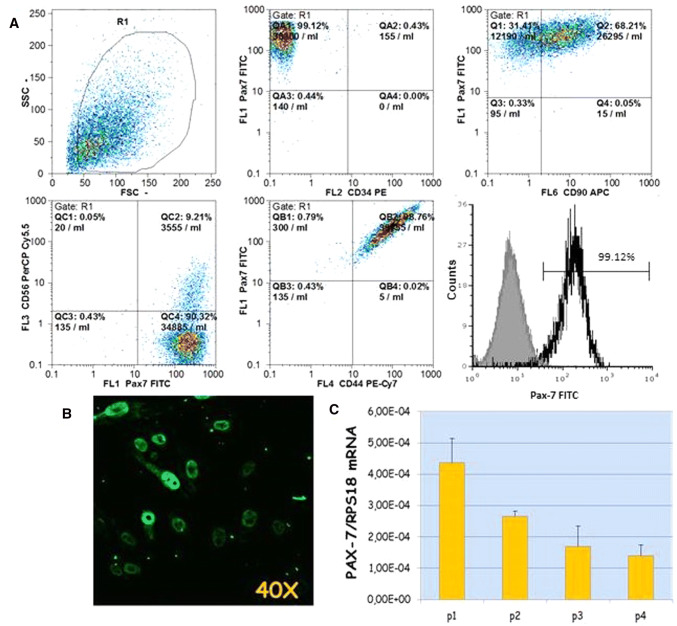
Expanded cells were subsequently characterized by flow cytometry in order to verify their phenotype, analyzing the cluster of differentiation (CD) marker surface proteins (CD44, CD90, CD105, CD56, CD34, CD45) and one of the most reliable markers of the satellite cells (PAX-7).
The phenotype analysis revealed that isolated hSkMCs expressed the surface markers CD44, CD90, CD105, commonly used to identify mesenchymal stem cells, with a very high percentage of positiveness. In contrast, the hematopoietic lineage marker CD45 was negative. Regarding PAX-7, analysis showed the presence of the nuclear transcription factor in 99.12% of total cells (Fig. 2a). On the contrary, CD34 and CD56, commonly used to identify satellite cells derived from mouse skeletal muscle, turned out to be non-specific for satellite cells derived from human skeletal muscle tissues; in fact, their presence on the cell surface is close to 0% and 9%, respectively (Fig. 2).
Fig. 2.
Scattergrams of phenotype characterization of hSkMCs by flow cytometry analysis. The overlay plot shows the percentage of hSkMCs expressing the nuclear marker PAX-7 (open histogram); grey histogram: autofluorescence of unstained cells (a); Observation in LSCM of the nuclear transcription factor of satellite cells PAX-7, objective ×40 (b); Real-Time qPCR of the PAX-7 gene during passages (from 1 to 4) of hSkMCs. Data are normalized for the housekeeping gene RPS18 (c)
Immunofluorescent staining of PAX-7, observed in LSCM, allowed the expression of the nuclear marker to be assessed. As expected from cytometry, it is clearly shown that the PAX-7 protein is present in the nuclei in all the expanded hSkMCs, confirming the isolation of human satellite cells (Fig. 2b).
Moreover, the gene expression of PAX-7 was analyzed over time, to verify its presence at cellular passages used for the experiments. As shown by Real-Time qPCR, we confirmed PAX-7 presence, but, as the passages increase, the gene expression of PAX-7 decreases, reducing the differentiating potential of cells (Fig. 2c). For that reason, we have limited the use of hSkMCs to passage 4.
Multipotentiality of hSkMCs
The multipotent evaluation of hSkMCs was assessed by the induction toward the adipogenic, osteogenic and myogenic phenotypes, using appropriate media described in “Materials and Methods” section.
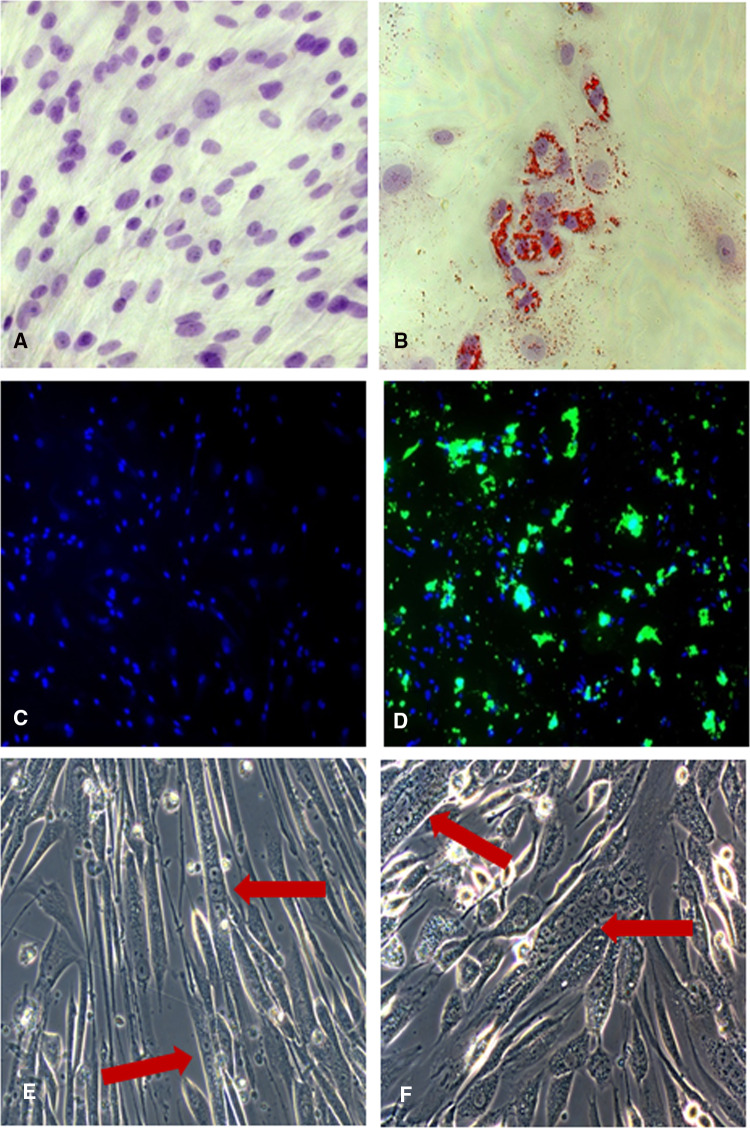
Adipogenic differentiation was performed culturing the hSkMCs in AM for 7 days, and confirmed, using Oil Red O staining, by the multiple intracellular lipid-filled droplets accumulation and microscopic observations in brightfield. In contrast, control cells grown in GM for the same time did not show any formation of lipid droplets (Fig. 3a, b).
Fig. 3.
Adipogenic phenotype evaluation of the hSkMCs. Representative images of the adipogenic phenotype evaluation of skeletal muscle-derived cells at time 0 (a) and after 7 days of induction (b). The intracellular lipid droplets are stained in red by Oil Red O, and nuclei counterstained with Mayer’s acid hemalum in blue. Images acquired in brightfield microscopy. Objective ×20. Osteogenic phenotype evaluation of the hSkMCs. Representative images of calcium staining at time 0 (c) and after 15 days of osteogenic induction (d). The mineralized calcium deposits are in fluorescent green and nuclei counterstained with propidium iodide in conventional blue color. Images acquired in epifluorescence microscopy. Objective ×10. Representative images in phase contrast microscopy of the multinucleated cells after 9 days of myogenic induction (e, f). The arrows show the formed myotubes. Objectives ×20
Osteogenic induction of hSkMCs was assessed with OM up to 15 days and observed monitoring the production of the mineralized calcium deposits, thanks to the fluorophore calcein added to the medium. Epifluorescence microscopic observations have shown calcein uptake in the calcium nodules after 15 days of osteogenic induction; in contrast, the control cells grown in GM for the same time did not show any deposition of calcium deposits (Fig. 3c, d).
Afterwards, the hSkMCs were differentiated toward the myogenic phenotype using DM for 9 days. During this period, cells started to approach one another, fusing with one another. Observations in phase contrast microscopy have revealed the presence of multinucleated elongated cells (containing from 3 to more than 8 nuclei) referable to myotubes (Fig. 3e, f).
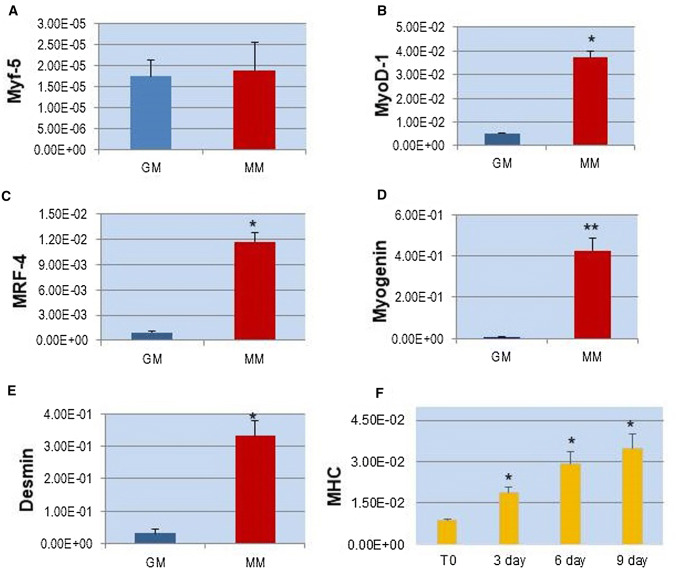
In order to confirm the myogenic induction of hSkMCs, we have performed, using Real-Time qPCR, the analysis of the MRFs (MyoD-1, Myf-5, MRF-4, Myogenin), the main myogenic differentiation genes, Desmin and MHC, after cultivating cells in MM up to 9 days. The results have shown a significant increase in the expressions of these genes during myogenesis, except for Myf-5, with respect to the control in GM (Time 0), indicating that differentiation occurred (Fig. 6). Moreover, differentiation has been supported by the presence of significant increases in the gene expression of Desmin and MHC, which represent essential proteins for proper muscular structure and function (Fig. 4).
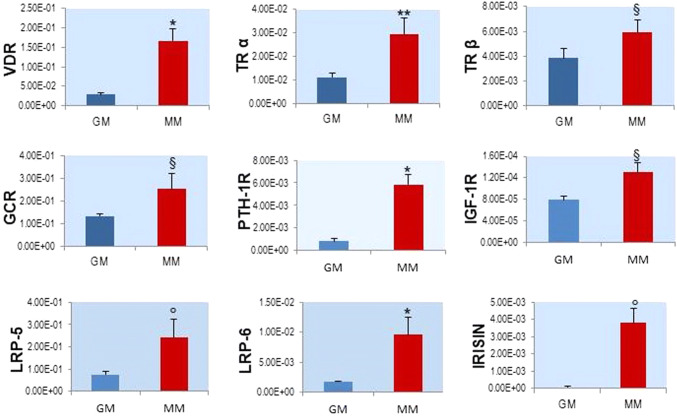
Fig. 6.
Real-Time qPCR of the hormone receptor genes during in vitro myogenesis of hSkMCs, in proliferation (red) and after 9 days of myogenic induction in DM (blue). Values are the mean ± SD of 3 independent experiments and they are expressed as the number of mRNA molecules of the genes normalized to RPS18 mRNA. *p < 0.001, **p < 0.005, °p < 0.01, §p < 0.05 versus control group in GM
Fig. 4.
Real-Time qPCR analysis of the main genes during in vitro myogenesis of hSkMCs, in proliferation (red), and after 9 days of myogenic induction in DM (blue). Values are the mean ± SD of 3 independent experiments and they are expressed as the number of mRNA molecules of the genes normalized to the housekeeping RPS18 mRNA. *p < 0.001, **p < 0.005 versus control group in GM
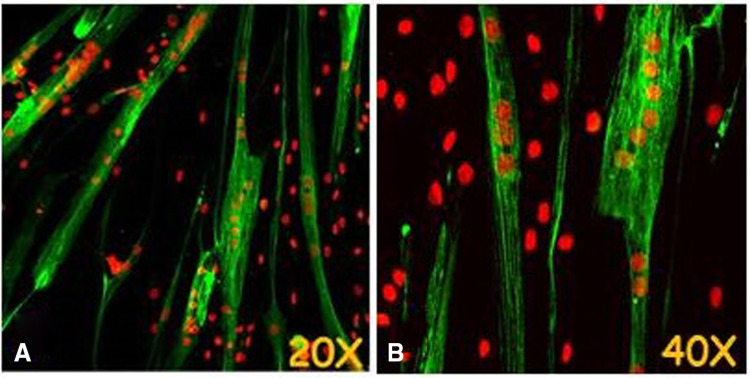
In particular, MHC was assessed at different time points (T0, 3, 6, 9 days) in order to follow the effective myogenesis over time (Fig. 4f). Since MHC is one of the most important proteins in skeletal muscle, and it is essential for contraction and muscle movement, it was analyzed by immunofluorescence staining. The microscopic observation of hSkMCs after 9 days in MM has shown the presence of MHC, demonstrating the suitability of our in vitro myogenesis model (Fig. 5).
Fig. 5.
Microscopic observations in LSCM of MHC in hSkMCs after 9 days of myogenic induction (Alexa Fluor 488, conventional green color). Nuclei counterstained with propidium iodide in conventional red color. Objective ×20 (a) and ×40 (b)
Gene Expression Analysis of Hormone Receptors in hSkMCs During In Vitro Myogenesis
The hSkMCs were differentiated using the MM and, after 9 days of induction, we analyzed the gene expression of the hormone receptors, in order to characterize the maturation and endocrine properties of the cellular model during myogenesis. In particular, we analyzed the following genes by Real-Time qPCR: VDR, TRα, TRβ, GCR, PTH-1R, IFG-1R, LRP-5, LRP-6 and Irisin.
The results of the hormone receptors analyzed have shown significant increases in gene expressions during cell differentiation with respect to the control group in growth medium, demonstrating the formation of the skeletal muscle as an endocrine apparatus during myogenesis (Fig. 6). Subsequently, the expression of Irisin, a hormone secreted by skeletal muscle, specifically suggests the development and maturation of new myofibers, since it represents a myokine secreted by mature endocrine tissue.
Discussion
The last decade has been an exciting period for the study of the biology of skeletal muscle stem cells and tissue regeneration and the development of novel human in vitro cell models can contribute to the identification of new mechanisms that control myogenesis [14–20]. These human cell cultures appear more suitable for predictive screening strategies when compared to rodent cell lines, such as C2C12 or rat L6 myoblasts [21, 22].
In this study, we have isolated and characterized skeletal muscle-derived cells from human biopsies to be used for the in vitro study of myogenesis. The developed cellular model was enriched in satellite cells, as confirmed by analyzing the presence of PAX-7 in more than 99% of the hSkMCs and the disappearance of the PAX-7 gene with each passage in culture [23]. For this reason, we decided to use cells not over the fourth passage in vitro.
Although CD34 antigen is a marker of satellite cells in murine models, our results indicate that, in human SCs, CD34 expression is not the hallmark of SCs, as it is present in only 0.5% of hSkMCs [24]. The same is true for CD56 antigen, which is present in only 9% of the isolated hSkMCs, a result that may be controversial considering other data in the literature, since it also marks natural killer lymphocytes [25, 26]. Flow cytometry analysis in hSkMCs has permitted the verification of the presence of the principal markers to be expressed on the surface of mesenchymal stem cells, specifically CD44, CD105 and CD90, and the negativity of the hematopoietic CD45 antigen, confirming the mesenchymal stemness of our cells, as largely recognized in the literature. The multipotentiality of the isolated hSkMCs was confirmed by demonstrating their own capacity to differentiate into the adipogenic, osteogenic and myogenic phenotypes, as assessed by cytochemical staining performed on cells, properly induced with specific differentiating media, as reported in the literature [27].
Using appropriate differentiation medium with confluent cells at 70–80% density in the plate, we were able to induce the alignment of the activated cells (myoblasts), the subsequent fusion with each other and, finally, the differentiation into multinucleated myofibers. Gene expression analyses have shown significant increases of MRFs, MyoD-1, MRF-4, and Myogenin, 9 days after myogenic induction which, together with Desmin and MHC gene expression augmentation during the entire study period, have confirmed myoblast determination and muscle differentiation, confirming the suitability of our in vitro myogenesis model. The only non-significance increase in gene expression was detected for the Myf-5 gene, in agreement with data reported in the literature. The reason may the fact that this transcription factor is the main controller of the activation of SCs toward myogenic differentiation and is expressed in a majority of quiescent SCs in adult muscle [3]. It is also reported that Myf-5, in the absence of MyoD-1, MRF-4 and Myogenin during development, is unable to drive myogenic differentiation, so Myf-5 may contribute to controlling "stemness" in the niche [4] [28].
Once validated the in vitro model, we embarked in the project of characterization of the endocrine machinery in the different stages of myocytic differentiation. This analysis was never performed before in in vitro models. The analysis was focused on selected receptor genes known to mediate a specific hormonal action on skeletal muscle tissue or suggested to mediate an endocrine action on skeletal muscles: VDR, TRα, TRβ, IGF-1R, PTH-1R, GCR, LRP-5 and LRP-6.
In the literature, it has been reported that vitamin D deficiency is a condition associated with skeletal muscle weakness and small muscle fiber size [29]. In animal models, the skeletal muscle dysfunction observed in vitamin D deficiency is reversed by vitamin D repletion, whereas vitamin D supplementation in humans has been found to increase skeletal muscle strength [30]. Many reports suggest that the VDR is expressed in skeletal muscle [31]. VDR deletion in mice results in alterations in muscle function and strength [32], and its association with interleukin-6 may play a role in intramuscular inflammation [33].
The thyroid hormone plays an essential role in myogenesis; it acts as a pleiotropic factor during development and regulates genes involved in growth and differentiation [34, 35]. In particular, data on C2C12 cells and primary myoblasts from mice have suggested the essential role of TRα in the optimal fusion and regeneration of myofibers after muscle injury and to maintain the SC niche during aging [36, 37]. Moreover, it has been reported that TRα is the dominant isoform thyroid receptor in C2C12 and murine primary myoblasts. This is in agreement with our results in which mRNA expression is higher for TRα with respect to TRβ.
Insulin-like Growth Factor-1 (IGF-1), the mediator of growth hormone function, strongly promotes the proliferation and differentiation of skeletal myoblasts. The anabolic effects of IGF-1 are mediated through specific binding with IGF-1R to promote the activation of the PI3K/art/mTOR signaling pathway, which is associated with protein synthesis and muscle hypertrophy [38, 39]. Moreover, IGF-1R is required for normal muscle growth, and its loss on mouse muscle leads to increased basal glucose uptake due to increases in levels of Glu1 and Glu4 transporters, chronic activation of Akt and AMPK signaling, and a loss of TBC1D1, data confirmed also in L6 myotubes [40].
Regarding parathyroid hormone (PTH) and its receptor (PTH-1R), very few data in the literature are reported about their effects on skeletal muscle cells. PTH has been shown to enhance the differentiation of mesoderm to various cell types, including osteoblasts and smooth muscle cells [41, 42]. Since skeletal muscle cells are derived from the mesoderm, it is conceivable that PTH may also influence the differentiation of these cells. It has been reported that PTH and the expression of PTH-1R accelerate the differentiation of SCs to myotubes in a mouse model [43].
Skeletal muscle is a notable target for glucocorticoids (GCs) in health and disease. GCs convey their signals mainly through an intracellular glucocorticoid receptor (GCR). Chronically increased levels of endogenous or exogenous GCs can lead to proteolysis, muscle wasting, myopathy, and induce insulin resistance with severe perturbation in systemic energy metabolism, while short exposures to high GC concentrations have been involved in the development of crucial illness myopathy [44, 45]. Despite its importance, data on GC signaling during human skeletal muscle regeneration and how GCR primary target genes confer metabolic function of GCs remain incomplete [46]. In the literature, it has been reported that GCR is involved in a positive regulation of muscle regulatory gene Myf-5 in the mouse myogenic cell line C2 [47].
LRP-5 and LRP-6 are highly homologous proteins with key functions in canonical Wnt signaling. Alteration in genes encoding these receptors or their interacting proteins is linked to human diseases and, for that reason, they have been a major focus of drug development efforts to treat several human conditions, including osteoporosis, cancer and metabolic disease [48]. Sclerostin is a circulating osteocyte-derived glycoprotein produced by the osteocytes that in a paracrine fashion negatively regulates Wnt signaling after binding the LRP5/LRP6 co-receptors in osteoblastic cells and its pharmacologic inhibition produces bone anabolic effects [49]. Conversely, endocrine effects of sclerostin on muscle morphology remain unknown, and very little data are reported in the literature [50].
In the present study, the genes encoding the receptors for the above outlined hormones were detected in a limited number of collected hSkMCs. Moreover, all the assayed receptor genes significantly increase during in vitro myogenesis of hSkMCs, supporting their role in the maturation of the human skeletal muscle. The fact that the two sclerostin receptors LRP-5 and LRP-6 are expressed in hSkMCs is opens for this important osteocytic protein a function as a hormone in the reciprocal interaction between bone and skeletal muscle. Interestingly, also the expression of the gene encoding Irisin, an important hormone produces by mature skeletal muscle tissue that affects cortical bone [51] increases during the process of in vitro differentiation of the hSkMCs.
Conclusions
In conclusion, our results have demonstrated the utility of skeletal muscle satellite cells, isolated from human biopsies, as an in vitro cell model to study the myogenesis process and the characterization of the skeletal muscle as an endocrine apparatus and a target organ for several hormones. In fact, all the assayed hormone receptor genes may represent feasible targets during skeletal muscle regeneration to be modulated throughout the skeletal muscle differentiation pathway. Similarly, muscle function should be evaluated in patients suffering of various endocrinopathies, a problem not routinely carried out by the endocrinologists. These findings could stimulate further research for a better understanding of disorders associated with impaired adult myogenesis, to help identify novel therapeutic interventions for these conditions. Future developments of this research will include the enlargement of the culture collection and the characterization of the novel evidenced targets in human skeletal myogenesis.
Acknowledgments
This study was funded and supported by the MIUR-PRIN2012_Muskendo (Protocol 2012227FLF) (to MLB), by the H2020, Excellent science, Marie Skłodowska—Curie Innovative Training Networks_CaSR Biomedicine (Grant Agreement No. 675228) (to MLB) and by the Fondazione Italiana sulla Ricerca per le Malattie dell'Osso (to MLB).
Abbreviations
- VDR
Vitamin D receptor
- TRα
Thyroid receptor α
- TRβ
Thyroid receptor β
- GCR
Glucocorticoid receptor
- PTH-1R
Parathyroid hormone receptor-1
- IFG-1R
Insulin-like growth factor 1 receptor
- LRP-5
Low-density lipoprotein receptor-related protein 5
- LRP-6
Low-density lipoprotein receptor-related protein 6
- PAX-7
Paired box protein 7
- MRFs
Myogenic regulatory factors
- MHC
Myosin heavy chain
- hSkMC
Human skeletal muscle-derived cells
- SCs
Satellite cells
Author Contribution
CR, RZ, LC, MLB conceived and designed the project; MI provided biopsies; CR, RZ developed the cellular model; CR, RZ, PS acquired the data; CR, RZ analyzed and interpreted the data; CR wrote the manuscript; MLB is guarantor. All authors have read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee AOU Careggi and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
All participants provided an informed written consent prior to inclusion.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cecilia Romagnoli, Email: cecilia.romagnoli@unifi.it.
Roberto Zonefrati, Email: roberto.zonefrati@unifi.it.
Preeti Sharma, Email: preeti.sharma@unifi.it.
Marco Innocenti, Email: marco.innocenti@unifi.it.
Luisella Cianferotti, Email: luisella.cianferotti@unifi.it.
Maria Luisa Brandi, Email: marialuisa.brandi@unifi.it.
References
- 1.Wang Y, Rudnicki M. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol. 2011;13:127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- 2.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tedesco F, Dellavalle A, Diaz-Manera J, et al. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120(1):11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zammit P. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Shan T. The role of satellite and other functional cell types in muscle repair and regeneration. J Muscle Res Cell Motil. 2019 doi: 10.1007/s10974-019-09511-3. [DOI] [PubMed] [Google Scholar]
- 6.Pederson B. Muscle as a secretory organ. Compr Physiol. 2013;3:1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 7.Yoon J, Kim J, Song P, et al. Secretomics for skeletal muscle cells: a discovery of novel regulators? Adv Biol Regul. 2012;52:340–350. doi: 10.1016/j.jbior.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Ohlendieck K. Proteomic identification of biomarkers of skeletal muscle disorders. Biomark Med. 2013;7:169–186. doi: 10.2217/bmm.12.96. [DOI] [PubMed] [Google Scholar]
- 9.Romagnoli C, Pampaloni B, Brandi ML. Muscle endocrinology and its relation with nutrition. Aging Clin Exp Res. 2019;31(6):783–792. doi: 10.1007/s40520-019-01188-5. [DOI] [PubMed] [Google Scholar]
- 10.Juhas M, Ye J, Bursac N. Design, evaluation, and application of engineered skeletal muscle. Methods. 2016;99:81–90. doi: 10.1016/j.ymeth.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romagnoli C, Zonefrati R, Puppi D, Rosati C, Aldinucci A, et al. Human adipose tissue-derived stem cells and a poly(ε-caprolactone) scaffold produced by computer-aided wet spinning for bone tissue engineering. J Biomater Tissue Eng. 2017;7(8):622–633. doi: 10.1166/jbt.2017.1614. [DOI] [Google Scholar]
- 12.Romagnoli C, Zonefrati R, Galli G, Puppi D, et al. In vitro behavior of human adipose tissue-derived stem cells on poly(ε-caprolactone) film for bone tissue engineering applications. Biomed Res Int. 2015;2015:323571. doi: 10.1155/2015/323571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nardone V, Zonefrati R, Mavilia C, Romagnoli C, Ciuffi S, et al. In vitro effects of strontium on proliferation and osteoinduction of human preadipocytes. Stem Cells Int. 2015;2015:871863. doi: 10.1155/2015/871863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nehlin JO, Just M, Rustan AC, Gaster M. Human myotubes from myoblast cultures undergoing senescence exhibit defects in glucose and lipid metabolism. Biogerontology. 2011;12:349–365. doi: 10.1007/s10522-011-9336-5. [DOI] [PubMed] [Google Scholar]
- 15.Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Krarlund Pedersen B, Scheele C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol. 2011;111:251–259. doi: 10.1152/japplphysiol.01399.2010. [DOI] [PubMed] [Google Scholar]
- 16.Davegardh C, Broholm C, Perfilyev A, Henriksen T, García-Calzon S, Peijs L, Schioler Hansen N, Volkov P, Kjobsted R, Wojtaszewski JFP, Pedersen M, Krarlund Pedersen B, Ballak DB, Dinarello CA, Heinhuis B, Joosten LAB, Nilsson E, Vaag A, Scheele C, Ling C. Abnormal epigenetic changes during differentiation of human skeletal muscle stem cells from obese subjects. BMC Med. 2017;15:39–65. doi: 10.1186/s12916-017-0792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddle ES, Bender EL, Thalacker-Mercer AE. Transcript profile distinguishes variability in human myogenic progenitor cell expansion capacity. Physiol Genomics. 2018;50:817–827. doi: 10.1152/physiolgenomics.00041.2018. [DOI] [PubMed] [Google Scholar]
- 18.Riddle ES, Bender EL, Thalacker-Mercer AE. Expansion capacity of human muscle progenitor cells differs by age, sex, and metabolic fuel preference. Am J Physiol Cell Physiol. 2018;315:C643–C652. doi: 10.1152/ajpcell.00135.2018. [DOI] [PubMed] [Google Scholar]
- 19.Gheller BJ, Blum J, Souied-Baumgarten S, Bender E, Cosgrove BD, Thalacker-Mercer A. Isolation, culture, characterization, and differentiation of human muscle progenitor cells from the skeletal muscle biopsy procedure. JOVE. 2019;150:e59580. doi: 10.3791/59580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kugler BA, Gundersen AE, Li J, Deng W, Eugene N, Gona PN, Houmard JA, Zou K. Roux-en-Y gastric bypass surgery restores insulin-mediated glucose partitioning and mitochondrial dynamics in primary myotubes from severely obese humans. Int J Obesity. 2019 doi: 10.1038/s41366-019-0469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultan K, Henkel B, Terlou M, Haagsman H. Quantification of hormone-induced atrophy of large myotubes from C2C12 and L6 cells: atrophy-inducible and atrophy-resistant C2C12 myotubes. Am J Physiol Cell Physiol. 2006;290:C650–C659. doi: 10.1152/ajpcell.00163.2005. [DOI] [PubMed] [Google Scholar]
- 22.Cui Z, Chen X, Lu B, Park S, et al. Preliminary quantitative profile of differential protein expression between rat L6 myoblasts and myotubes by stable isotope labeling with amino acids in cell culture. Proteomics. 2009;9:1274–1292. doi: 10.1002/pmic.200800354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama S, Asahara H. The myogenic transcriptional network. Cell Mol Life Sci. 2011;68(11):1843–1849. doi: 10.1007/s00018-011-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bareja A, Holt J, Luo G, Chang C, et al. Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis. PLoS ONE. 2014;9(2):e90398. doi: 10.1371/journal.pone.0090398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trapecar M, Kelc R, Gradisnik L, Vogrin M, Rupnik M. Myogenic progenitors and imaging single-cell flow analysis: a model to study commitment of adult muscle stem cells. J Muscle Res Cell Motil. 2014;35(5–6):249–257. doi: 10.1007/s10974-014-9398-5. [DOI] [PubMed] [Google Scholar]
- 26.Illa I, Leon-Monzon M, Dalakas M. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992;31(1):46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- 27.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 28.Valdez M, Richardson J, Klein W, Olson E. Failure of Myf5 to support myogenic differentiation without myogenin, MyoD, and MRF4. Dev Biol. 2000;219(2):287–298. doi: 10.1006/dbio.2000.9621. [DOI] [PubMed] [Google Scholar]
- 29.Girgis C, Clifton-Bligh R, Hamrick M, Holick M, Gunton J. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 30.Beaudart C, Buckinx F, Rabenda V, Gillain S, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–4345. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Kishimoto K, Okuno H, Saito H, Itoi E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve. 2014;49(5):700–708. doi: 10.1002/mus.23950. [DOI] [PubMed] [Google Scholar]
- 32.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144(12):5138–5144. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 33.Pojednic R, Ceglia L, Lichtenstein A, Dawson-Hughes B, Fielding R. Vitamin D receptor protein is associated with interleukin-6 in human skeletal muscle. Endocrine. 2015;49(2):512–520. doi: 10.1007/s12020-014-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Kim N, Milanesi A. Thyroid hormone signaling in muscle development, repair and metabolism. J Endocrinol Diabetes Obes. 2014;2(3):1046. [PMC free article] [PubMed] [Google Scholar]
- 35.Bloise F, Oliveira T, Cordeiro A, Ortiga-Carvalho T. Thyroid hormones play role in sarcopenia and myopathies. Front Physiol. 2018;9:560. doi: 10.3389/fphys.2018.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milanesi A, Lee J, Kim N, Liu Y, et al. Thyroid hormone receptor α plays an essential role in male skeletal muscle myoblast proliferation, differentiation, and response to injury. Endocrinology. 2016;157(1):4–15. doi: 10.1210/en.2015-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milanesi A, Lee J, Yang A, Liu Y, Sedrakyan S, et al. Thyroid hormone receptor alpha is essential to maintain the satellite cell niche during skeletal muscle injury and sarcopenia of aging. Thyroid. 2017;27(10):1316–1322. doi: 10.1089/thy.2017.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Retamales A, Zuloaga R, Valenzuela C, Gallardo-Escarate C, et al. Insulin-like growth factor-1 suppresses the Myostatin signaling pathway during myogenic differentiation. Biochem Biophys Res Commun. 2015;464(2):596–602. doi: 10.1016/j.bbrc.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Glass D. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267–278. doi: 10.1007/82_2010_78. [DOI] [PubMed] [Google Scholar]
- 40.O'Neill B, Lauritzen H, Hirshman M, Smyth G, et al. Differential role of insulin/IGF-1 receptor signaling in muscle growth and glucose homeostasis. Cell Rep. 2015;11(8):1220–1235. doi: 10.1016/j.celrep.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Wang M, Li Y, Li C, et al. The PERK-EIF2α-ATF4 signaling branch regulates osteoblast differentiation and proliferation by PTH. Am J Physiol Endocrinol Metab. 2019;316(4):E590–E604. doi: 10.1152/ajpendo.00371.2018. [DOI] [PubMed] [Google Scholar]
- 42.Song G, Fiaschi-Taesch N, Bisello A. Endogenous parathyroid hormone-related protein regulates the expression of PTH type 1 receptor and proliferation of vascular smooth muscle cells. Mol Endocrinol. 2009;23:1681–1690. doi: 10.1210/me.2009-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura S, Yoshioka K. Parathyroid hormone and parathyroid hormone type-1 receptor accelerate myocyte differentiation. Sci Rep. 2014;4:5066. doi: 10.1038/srep05066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menconi M, Gonnella P, Petkova V, Lecker S, Hasselgren P. Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J Cell Biochem. 2008;105(2):353–364. doi: 10.1002/jcb.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filipović D, Pirkmajer S, Mis K. Mars T and Grubic Z (2011) Expression of glucocorticoid receptors in the regenerating human skeletal muscle. Physiol Res. 2011;60(1):S147–S154. doi: 10.33549/physiolres.932171. [DOI] [PubMed] [Google Scholar]
- 46.Kuo T, Harris C, Wang J. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol. 2013;380(1–2):79–88. doi: 10.1016/j.mce.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Auradé F, Pfarr C, Lindon C, Garcia A, et al. The glucocorticoid receptor and AP-1 are involved in a positive regulation of the muscle regulatory gene myf5 in cultured myoblasts. J Cell Sci. 1997;110(Pt 22):2771–2779. doi: 10.1242/jcs.110.22.2771. [DOI] [PubMed] [Google Scholar]
- 48.Joiner D, Ke J, Zhong Z, Xu H, Williams B. LRP5 and LRP6 in development and disease. Trends Endocrinol Metab. 2013;24(1):31–39. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moester M, Papapoulos S, Löwik C, van Bezooijen R. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010;87(2):99–107. doi: 10.1007/s00223-010-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips E, Beggs L, Ye F, Conover C, et al. Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PLoS ONE. 2018;13(3):e0194440. doi: 10.1371/journal.pone.0194440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colaianni G, Cinti S, Colucci S, Grano M. Irisin and musculoskeletal health. Ann N Y Acad Sci. 2017;1402(1):5–9. doi: 10.1111/nyas.13345. [DOI] [PubMed] [Google Scholar]