Figure 1. Contribution of Nek2A degrons in binding and ubiquitination by the APC/C and Nek2A MR tail binding site on Apc8.
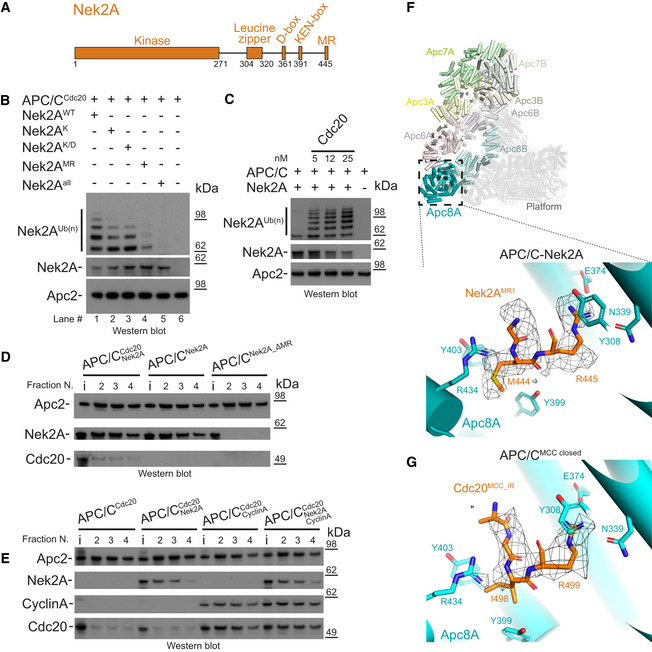
- Schematic representation of the domain and degron architecture of Nek2A.
- The MR tail is the main contributor for Nek2A ubiquitination. Ubiquitination reactions performed with APC/CCdc20 for Nek2A wild‐type (Nek2AWT) and degron mutants. Nek2AK is the KEN‐box mutant (391KEN393/KAA), Nek2AK/D is the (KEN‐ and D‐box mutant (391KEN393/KAA, 361RKFL364/AKFA), and Nek2AMR is the 443ΔMR mutant.
- The APC/C requires the Cdc20 coactivator subunit to ubiquitinate Nek2A. Nek2A ubiquitination reactions performed with the APC/C in the absence and presence of increasing concentrations of Cdc20 using the E2 UbcH10.
- The MR tail is essential for Nek2A binding to the APC/C, whereas in contrast Cdc20 is dispensable. Size‐exclusion chromatography peak fractions of APC/C complexes with Nek2A. The input material (13% of total) is indicated (i). Peak fractions are numbered in respect of the chromatograms in Fig EV1.
- Nek2A binding does not stabilize binding of the coactivator subunit Cdc20 to the APC/C and does not compete with cyclin A for binding to the APC/C. Size‐exclusion chromatography peak fractions of APC/C complexes with Nek2A.
- Top: Cylinder representation of the APC/C–Nek2A complex structure. The TPR lobe subunits are highlighted: Apc8 (cyan), Apc6 (pink), Apc3 (yellow) and Apc7 (green). The platform subunits and the small subunits from the TPR lobe are shown in transparency. Bottom: cryo‐EM density of the Nek2AMR tail dipeptide and details of the Nek2AMR tail (orange) binding site on Apc8A.
- Details of the IR tail of Cdc20MCC (orange) bound to Apc8A in APC/CMCC‐closed. This is the same site as the Nek2AMR tail binding site shown above. Shown is the cryo‐EM density for the Cdc20MCC IR tail and contacting residues of Apc8A.
Source data are available online for this figure.
