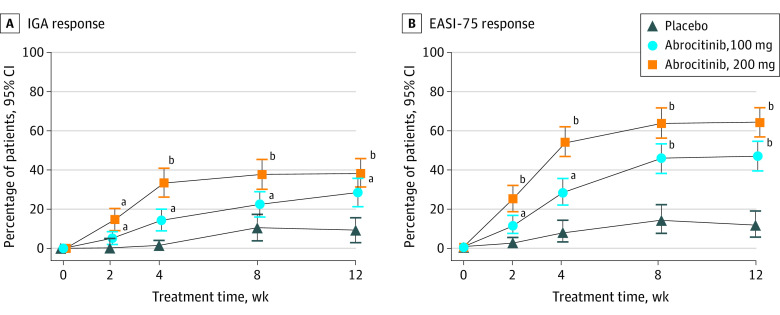
Figure 2. Coprimary End Points at Week 12.
Data are represented as the percentage of patients achieving Investigator Global Assessment (IGA) response (ie, clear [0] or almost clear [1], with improvement of ≥2 grades) and at least 75% improvement in Eczema Area and Severity Index (EASI-75) score from baseline. IGA response (A) was achieved in 38.1% of patients in the 200-mg group (59 of 155), 28.4% in the 100-mg group (44 of 155), and 9.1% in the placebo group (7 of 77). EASI-75 response (B) was achieved by 61.0% in the 200-mg group (94 of 154), 44.5% in the 100-mg group (69 of 155), and 10.4% in the placebo group (8 of 77). Error bars represent 95% CIs. Conclusion of statistical significance was controlled for multiplicity only at week 12.
aP < .05 vs placebo.
bP < .001 vs placebo.