Abstract
Glycine and methylamine are meteoritic water-soluble organic compounds that provide insights into the processes that occurred before, during, and after the formation of the Solar System. Both glycine and methylamine and many of their potential synthetic precursors have been studied in astrophysical environments via observations, laboratory experiments, and modeling. In spite of these studies, the synthetic mechanisms for their formation leading to their occurrence in meteorites remain poorly understood. Typical 13C-isotopic values (δ13C) of meteoritic glycine and methylamine are 13C-enriched relative to their terrestrial counterparts; thus, analyses of their stable carbon isotopic compositions (13C/12C) may be used not only to assess terrestrial contamination in meteorites, but also to provide information about their synthetic routes inside the parent body. Here, we examine potential synthetic routes of glycine and methylamine from a common set of precursors present in carbonaceous chondrite meteorites, using data from laboratory analyses of the well-studied CM2 meteorite Murchison. Several synthetic mechanisms for the origins of glycine and methylamine found in carbonaceous chondrites may be possible, and the prevalence of these mechanisms will largely depend on (a) the molecular abundance of the precursor molecules and (b) the levels of processing (aqueous and thermal) that occurred inside the parent body. In this work, we also aim to contextualize the current knowledge about gas-phase reactions and irradiated ice grain chemistry for the synthesis of these species through parent body processes. Our evaluation of various mechanisms for the origins of meteoritic glycine and methylamine from simple species shows what work is still needed to evaluate both, the abundances and isotopic compositions of simpler precursor molecules from carbonaceous chondrites, as well as the effects of parent body processes on those abundances and isotopic compositions. The analyses presented here combined with the indicated measurements will aid a better interpretation of quantitative analysis of reaction rates, molecular stability, and distribution of organic products from laboratory simulations of interstellar ices, astronomical observations, and theoretical modeling.
Keywords: Meteoritic organics, glycine, parent body processes, interstellar ice, methylamine
Graphical Abstarct
1. Introduction
Many carbonaceous chondrites, particularly the CI, CM, and CR groups that did not experience extensive parent body thermal alteration, contain a rich suite of primordial organics that may include compounds that formed before the Solar System, as well as compounds formed inside asteroidal parent bodies from presolar precursors.1–3 Amino acids are among the most well-studied organic compounds in carbonaceous chondrites.4–7 Conversely, the molecular distribution and isotopic composition of meteoritic amines have been only recently investigated,8–11 and their potential synthetic relationship with amino acids is not fully understood.
Glycine (NH2CH2CO2H) and methylamine (CH3NH2) are simple structurally analogous compounds related to each other by the presence or absence of the acid moiety (carboxyl group, - CO2H; Scheme 1). Both compounds are common in the terrestrial biosphere. Both have also been detected in multiple extraterrestrial samples, including carbonaceous chondrites,12–14 laboratory measurements of acid-hydrolyzed hot water extracts of comet-exposed materials from the Stardust sample return mission to comet 81P/Wild 2,15–17 and direct in situ measurements of the coma of comet 67P/Churyumov-Gerasimenko by the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis (ROSINA) instrument.18 Methylamine, but not glycine, was also identified by the Philae lander Cometary Sampling and Composition (COSAC) instrument shortly after Philae’s first contact with the surface of Churyumov-Gerasimenko.19 Additionally, glycine and methylamine have been produced from synthesis in gas-phase reactions,20–23 UV-irradiated interstellar ice analogs,24–32 and in Miller-Urey-type experiments,33–34 representing potential abiotic syntheses in diverse environments.
Scheme 1.
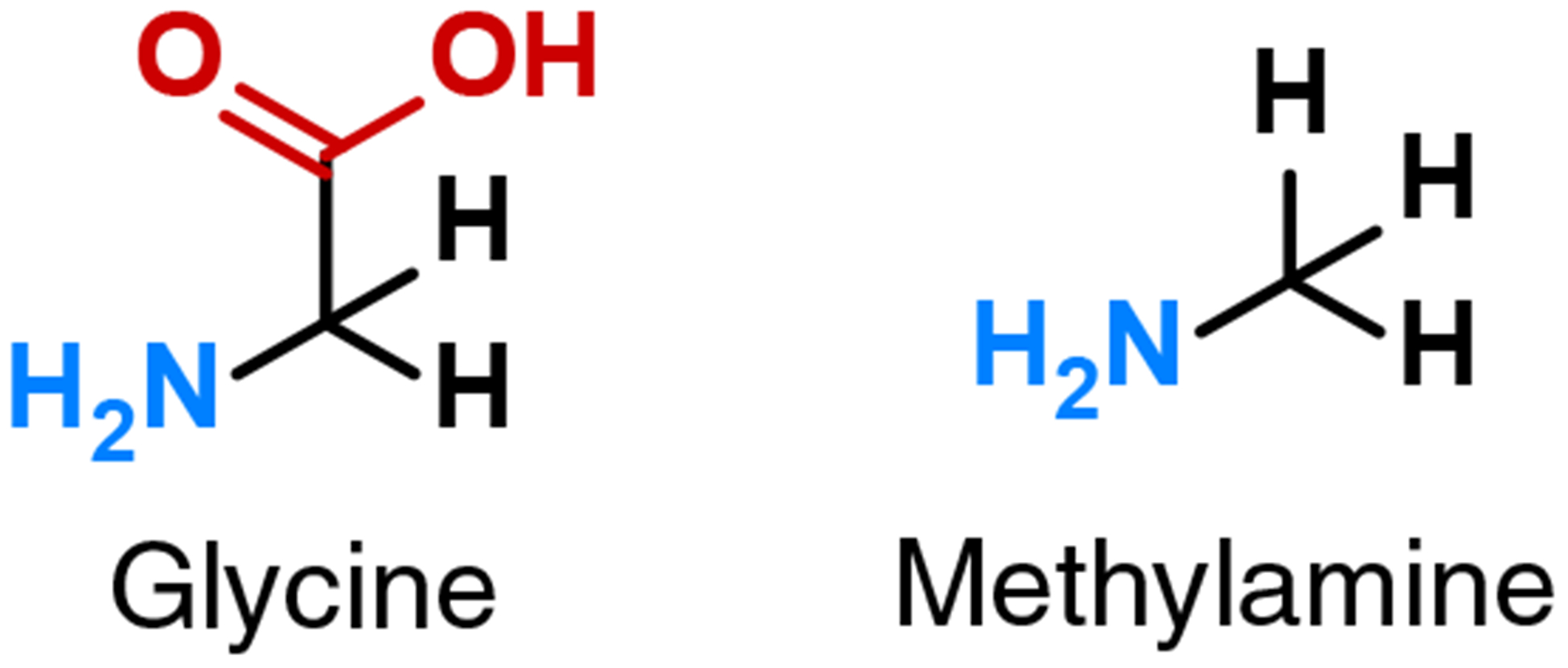
Structures of glycine and methylamine (color highlights the amine [blue, NH2] and acid [red, CO2H] functional groups).
Analysis of the 13C isotopic compositions of meteoritic organics and their precursors may provide insights about their synthetic origins. The synthesis of meteoritic glycine and methylamine may have occurred in two broad cosmochemical regimes, the first dominated by gas- and ice-grain chemistry that occurred in the molecular cloud, the solar nebula, or the protoplanetary disk, and the second dominated by hydrothermal chemistry inside the meteorite parent body; thus, δ13C values measured in the laboratory may result from isotopic fractionation inside various environments. Glycine and methylamine can be synthesized from common precursors such as carbon monoxide (CO), ammonia (NH3), hydrogen cyanide (HCN), and carbon dioxide (CO2); glycine and methylamine and their corresponding simpler building blocks may all have been incorporated during the accretion of Solar System bodies such as comets and asteroids, inside which further synthesis may have occurred.
Analyses of meteoritic δ13C values found for molecules regarded as precursors (CO, CO2, and HCN), and those of larger species such as glycine and methylamine (so-called products) extracted from carbonaceous meteorites suggest potential synthetic relationships between these molecules.35–37 The 13C-isotopic composition of the organic compounds found in carbonaceous chondrites and analyzed in the laboratory is the result of various processes that occurred before and after the accretion of the parent body. The degree of processing inside the parent body may have greatly shaped the δ13C signatures of the organic compounds evaluated in the laboratory. Indeed, the varying levels of aqueous and thermal processing for different carbonaceous asteroids and chondrite types and the conditions under which this processing occurred are currently poorly understood.38–41
Therefore, without being able to fully account for the level of fractionation that occurred through aqueous and thermal processing, it remains challenging to evaluate the synthetic routes leading to the origins of meteoritic glycine and methylamine. An additional challenge in using the δ13C values of meteoritic glycine and methylamine as probes to their formation mechanisms is the unknown original concentration and isotopic values of their potential precursor molecules inside carbonaceous chondrites. Being mindful of these limitations and with the available data currently present in the literature, here we examine plausible diverse synthetic pathways that may have led to the origins of glycine and methylamine in meteorites. We consider those processes that occurred before the accretion of the meteorite parent body, but center our attention on those synthetic mechanisms that may have taken place much later during the parent body stage which are dominated by hydrothermal reactions. We focus on the Murchison meteorite because is the most thoroughly studied carbonaceous chondrite for amino acids,7 as well as the only meteorite from which the stable carbon isotopic ratios (δ13C) of most of these potential precursor molecules have been reported. While other meteorites such as Orgueil (CI1), Lonewolf Nunataks (LON) 94101 (CM2), Lewis Cliff (LEW) 90500 (CM2), Allan Hills (ALH) 83100 (CM1/2), La Paz Icefield (LAP) 02342 (CR2), and Graves Nunataks (GRA) 95229 (CR2) contain glycine and methylamine,9,10 the abundances and δ13C of precursor molecules such as CO, CO2, and HCN in these meteorites have not been reported. Isotopic analyses of precursor molecules in additional meteorites are needed to test and expand the hypotheses presented in this manuscript. We also evaluate the current knowledge of gas-phase reactions, irradiated ice grain chemistry, theoretical modeling, and telescopic observations of various interstellar regions for the synthesis of glycine, methylamine, and their corresponding precursor species.
2. The Pre-Parent body Phase
Inside interstellar environments, complex organic compounds may form in grain-surface and gas-phase reactions by hydrogen atom addition to CO and other unsaturated molecules, followed by carbon atom addition;42–47 many of the predicted compounds such as formaldehyde and methanol, have been detected in the interstellar and circumstellar media, hot cores, interstellar clouds and circumstellar envelopes.48–50 Several molecules of interest here, which may form in both surface- and gas-phase reactions (such as formaldehyde, methanimine, and methylamine) have been detected in interstellar and protostellar sources,51–54 in comets,19,55–57 and in carbonaceous chondrites.12,14,35,58 Although glycine detection in the interstellar medium remains controversial,59–61 possible formation pathways relevant to these environments have yet to be confirmed.22,62–65 Similarly, even though methylamine and its structurally related compound, methanimine, are both observed in the interstellar medium,66–69 their pre-solar formation mechanisms are not entirely understood. For example, Suzuki et al. (2016) have surveyed several star-forming cores for methanimine (CH2NH) with the view that cores with the highest abundances would be the best places to conduct a subsequent glycine search.70 Suzuki et al. (2016) also modeled the related gas-grain chemistry and concluded that interstellar methanimine formed in gaseous reactions.70
Recent observations of nearby young stellar objects (YSOs) in star forming regions with infrared absorption spectroscopy, have measured a discrepancy between gas-phase 12CO/13CO and solid-phase 12CO/13CO ratios.71 The observed 12CO/13CO gas-phase ratios of the Solar System and local interstellar medium ranges from ~+290 to +370‰ which is higher,72 (more 13C enriched) than those measured in YSOs and nearby star-forming regions ranging at ~−460 to +47‰.71 This leads to speculation that this effect may be due to isotopologue partitioning between the two reservoirs. Smith et al. (2015) ruled out isotope selective photo-dissociation for their results of gas-phase 12CO/13CO, even though it is a significant effect for the oxygen isotopes.71 The evolution of these objects through the prestellar disk and eventually planetary systems however, provides ample opportunity for further isotope effects to occur. Observational instrumentation is just reaching more complex molecular (and isotopic) detection limits, especially spatially, of these more evolved systems,73 allowing for new detections and observational constraints to current theories. Models of disk chemistry suggest the fractionation to be dependent on the disk radius and height with some chemical dependence,74 though the mid-plane results are consistent with current observations of comets.
In the cold interstellar medium, 13C nuclei are effectively incorporated into 13CO while expelling 12C nuclei, through the following ion-molecule exchange reaction: 13C+ + 12CO ↔ 13CO + 12C+.75 Therefore, it is expected that the carbon atoms and other C-bearing molecules (“CX”, also known as “the carbon isotope pool”) are depleted in 13C and consequently have much higher 12CX/13CX ratios. Such depletion is therefore expected in other molecules formed primarily in gaseous reactions, such as HCN. Indeed, astronomical measurements using radio telescopes by Sakai et al. (2010)76 and Yoshida et al. (2015)77 have demonstrated the expected 13C-depletions in interstellar acetylene radical (C≡C-H) and cyclopropenylidene (c-C3H2). Conversely, formaldehyde has been tentatively found to be more 13C-enriched than CO in in some star-forming cores,78 a result that is contrary to all theoretical predictions and that suggest that isotopic partitioning has not yet been demonstrated unequivocally for observations of a large suite of interstellar molecules. Large observational errors are obtained in 12C/13C ratios measurements,79 which may not be very constraining on the ppm/ppb levels measured for organics in carbonaceous chondrites. Thus, a bulk isotope ratio cannot constrain the origin of meteoritic organics without extensively considering the chemistry and other isotope effects for each molecule present.
3. The Parent Body Phase
Chain-elongation reactions leading to the formation of more complex organic molecules may preferentially yield 12C-enriched products because breaking and creating 12C-12C bonds demands slightly less energy than breaking and creating 12C-13C bonds.35,80,81 Thus, it is expected that larger organic compounds will be 13C-depleted relative to their molecular precursors; the remaining unreacted starting materials would be 13C-enriched relative to the newly formed products and to their original compositions.
Table 1 shows the stable carbon isotopic measurements (in δ13C notations) of several molecular species that may serve as precursors or that share the same structural aliphatic backbone with glycine and methylamine. The δ13C values reported for HCN extracted from the Murchison meteorite shows that meteoritic HCN is 13C-rich relative to meteoritic CO extracted from the same carbonaceous chondrite.58 These results are difficult to understand based on interstellar fractionation alone, unless the 12CO and 13CO molecules underwent isotopic-selective photodissociation in the protosolar nebula producing a homogenous 12C/13C fractionation pattern,74 which is observed among the different meteoritic carbon species (Table 1). The δ13C of meteoritic organics shown in Table 1 average to δ13C ~35‰, this value is consistent with the Solar System δ13C value (~+25‰);72 however, it’s worth noticing that large errors are present from 12C/13C observational measurements (see Section 1). In the case of the isotopic difference between meteoritic HCN and CO however, the 13C nuclei could have been restored to the gas and eventually take part in chemical reactions in gas phase that enriched molecules of the carbon isotope pool in 13C. However, it is also possible that CO in Murchison may have been more readily outgassed or evaporated from nebular dust and planetesimals early in nebular evolution, and therefore further fractionated than the comparably less volatile HCN.
Table 1.
δ13C values of the molecular species evaluated here for the origins of glycine and methylamine in carbonaceous chondrites.
| Carbonaceous chondrite | Type | δ13C (%0) | ||||||
|---|---|---|---|---|---|---|---|---|
| CO | CO2 | CO32-(carbonate) | HCN | CH3CO2H (acetic acid) | CH3NH2 (methylamine) | NH2CH2CO2H (glycine) | ||
| Orgueil | CI1 | – | – | +60 ± 382, +59.2 ± 0.284, +68.8 ± 0.284 | – | – | +43 ± 109 | +2285 |
| ALH 83100 | CM1/2 | – | – | +44.5 ± 0.284 | – | – | +41 ±610 | +1189, +53 ± 310 |
| Murchison | CM2 | −32 ± 235 | +29.1 ±0.235 | +37 ± 382, +43.9 ±0.284 | +5 ± 258 | +22.7 ± 0.235, −7.7 ± 0.288 | + 129 ±78 | +2290, +41 ± 291, +13 ± 389 |
| LEW 90500 | CM2 | – | – | +41.3 ±0.284 | – | – | +59 ±810 | +47 ± 1089, +47 ± 110 |
| LON 94101 | CM2 | – | – | +41.3 ±0.284 | – | – | +44 ±610 | +38 ± 389, +36 ± 410 |
| LAP 02342 | CR2 | – | – | +36.2 ± 0.284 | – | – | +64.6 ± 1.611, + 10 ± 1310 | +20.1 ± 0.114 |
| GRA 95229 | CR2 | – | – | +42.0 ± 0.284 | – | – | +64.0 ± 2.111, −1 ± 910 | +33.8 ± 1.692, +35 ± 989 |
35Yuen et al. 1984. 82Grady et al. 1988. 84Alexander et al. 2015. 58The value shown here is the average of four different samples measured by Pizzarello (2014). 88Huang et al. 2005. 9Aponte et al. 2015. 10Aponte et al. 2016. 8Aponte et al. 2014. 11Pizzarello and Yarnes 2016. 85Ehrenfreund et al. 2001. 89Elsila et al. 2012. 90Engel et al. 1990. 91Pizzarello et al. 2004. 14Pizzarello and Holmes 2009. 92Martins et al. 2007.
Orgueil, one of the most aqueously altered carbonaceous chondrites, shows 13C-enriched carbonates relative to the other chondrite types (the origins of meteoritic carbonates are currently poorly understood);82–84 aqueous processes have been linked to the destruction of meteoritic organic species,85,86 which may potentially lead to isotopic fractionation. However, the different measurements of meteoritic compounds do not show a simple and direct relationship between the degrees of aqueous processing and the δ13C values as shown in Table 1, suggesting that the accretion of the parent body was highly heterogeneous. Indeed, CI, CM and CR meteorites likely originate from distinct parent bodies, which may have accreted different abundances of water and thus, may have resulted in different levels of processing and isotopic compositions (meteoritic water contents have been found to follow the trend: CI1 > CM1/2 > CM2 > CR2)87. Regardless, further δ13C measurements of these and other carbon sources in carbonaceous chondrites and experimental modeling mimicking conditions inside the parent body are needed to further our understanding of the synthetic pathways for meteoritic amines and amino acids.
3.1. Synthesis of glycine and methylamine from formaldehyde.
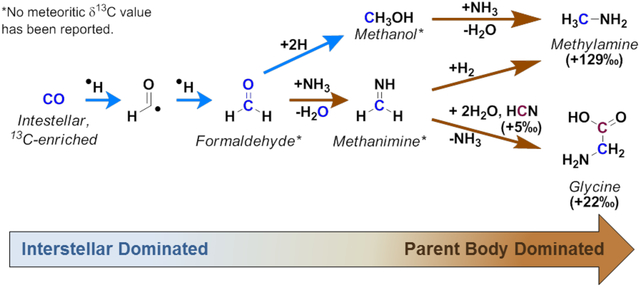
Two different synthetic routes which may interconnect the synthetic origins of glycine and methylamine prior to and after the formation of the asteroid parent body are shown in Scheme 2. The synthesis of interstellar complex organics may start from the hydrogenation of interstellar CO to form formaldehyde;43,45,46 this first step should occur prior to the accretion of the asteroid inside the interstellar medium and later in the protosolar nebula and protoplanetary disk midplane, since the radical intermediate in the hydrogenation of CO may react rapidly with surrounding water and mineral species present inside the parent body, quenching or inducing the loss (via polymerization) of this intermediate product. After interstellar formaldehyde is formed, it may have accreted into the parent body, where it could react with surrounding ammonia to form methanimine. Ammonia has been reported from various carbonaceous meteorites including Murchison (CM2) and the CR2 chondrites LAP 02342 and GRA 95229; those results indicated that ammonia is two orders of magnitude less abundant in Murchison than in the CR2 chondrites.13,93 Indeed, the abundance of amino acids has been found to be about ten times larger in CR2 chondrites than in the Murchison meteorite, suggesting that larger concentrations of ammonia may be linked to larger amino acid abundances in carbonaceous chondrites.14,94 Continuing with the synthesis of meteoritic glycine, methanimine may readily react with meteoritic HCN and form α-aminoacetonitrile (Strecker-cyanohydrin reaction), which yields the amino acid after aqueous hydrolysis (Scheme 2). Laboratory simulations of the reactivity of aminoacetonitrile in ice analogs by Danger et al. (2011a) did not find evidence for the formation of glycine at temperatures ranging from 20 to 300 K, suggesting that the formation of glycine would result after thermal activation inside the parent body.95
Scheme 2.
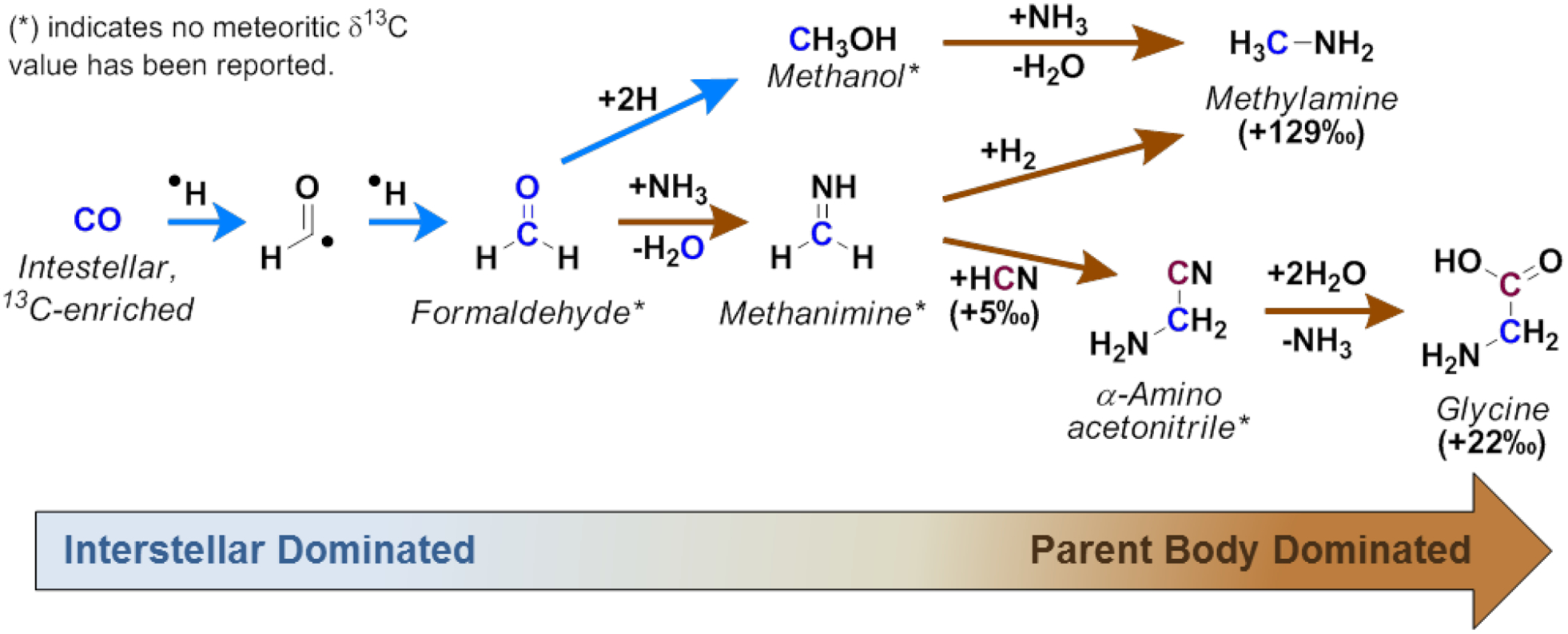
The Strecker-cyanohydrin synthesis and reductive amination of formaldehyde are potential synthetic routes for glycine and methylamine in the Murchison meteorite. Starting from 13C-enriched CO, results in 13C-enriched glycine and methylamine. These reactions may be dominant, but not exclusive inside the indicated environments.
The Strecker-cyanohydrin synthesis may be driven by parent-body aqueous processes, and is the most commonly invoked mechanism for the production of meteoritic α-amino acids such as glycine inside the parent body.96,97 As shown in Scheme 2, one molecule of methanimine (formed from formaldehyde) reacts with one molecule of HCN to yield the α-aminonitrile. Again, it would be expected that formaldehyde and methanimine are 13C-enriched relative to HCN (occurring from the carbon isotope pool; see Section 1) inside the parent body, and that the δ13C composition of glycine before extended parent body processing would be the result of the combination of these two species. However, the preaccretionary abundance and δ13C composition of formaldehyde, methanimine, and HCN upon accretion into the meteorite parent body, as well as the effects of parent body processing on the abundances and isotopic fractionation of these meteoritic species remains unknown. Without this information, it is impossible to confirm the roles of these proposed starting materials as glycine precursors; thus, both measurements of the δ13C values of meteoritic formaldehyde and methanimine, as well as a more thorough understanding of the effects of parent body processing over the δ13C composition of meteoritic organics are necessary future investigations to constrain the formation of meteoritic glycine.
Similar to glycine, methylamine may be synthesized from formaldehyde but through the hydrogenation of methanimine (Scheme 2); indeed, the hydrogenation of methanimine to yield methylamine has been experimentally tested in interstellar ice analogs with positive results.30,31 It remains to be seen, however, whether this reaction would be viable inside an asteroid-like environment. During the synthesis of glycine through this reaction, unreacted methanimine would become 13C-enriched relative to the produced glycine. Methylamine extracted from the acid-hydrolyzed extract of the Murchison meteorite showed a δ13C value which is 13C-enriched relative to the isotopic values reported for glycine (Table 1). Therefore, the δ13C relationship between meteoritic glycine and methylamine may suggest that methylamine could have formed from hydrogenation of methanimine left unreacted from the synthesis of glycine (Scheme 2). Similar to Murchison, methylamine was more 13C-enriched than glycine in the LEW 90500, LON 94101, and Orgueil meteorites, although opposite results were observed in the CM1/2 chondrite ALH 83100 and two CR2 chondrites (Table 1). Therefore, this synthetic pathway may have to be reevaluated after future efforts to expand the δ13C analyses of glycine and methylamine to other carbonaceous chondrites from different petrologic types.
An alternative synthetic route for obtaining meteoritic methylamine may arise from the formation of methanol after reduction of formaldehyde (Scheme 2). Through the reduction of formaldehyde inside meteorites, protonated methanol would undergo dehydration upon reaction with ammonia. The synthesis of aliphatic amines from the reaction of alcohols and ammonia has been observed in high yields in the presence of transition metals, all of which may be present inside the asteroid parent body, e.g., iron, nickel, phyllosilicates; however, the production of tertiary amines as main products of this reaction may be a factor to consider.98–100 Still, evaluation of the meteoritic δ13C values of methanol and formaldehyde is needed to link their synthetic relationship to meteoritic methylamine.
3.2. Synthesis of glycine from CO2 or HCN addition to methylamine.
An alternative route for the synthesis of meteoritic glycine is the addition of CO2 (which may have nebular origins or may be generated from the reaction of carbonates and proton donors inside the parent body), or HCN to methylamine inside the parent body (Scheme 3). Experimental investigations of this ion/neutral reaction in the gas phase have been unsuccessful, yielding only proton transfer products rather than the amino acid;100 however, the reaction may occur in ice chemistry. The synthesis of amino acids from the addition of CO2 to aliphatic amines has been demonstrated from photochemical and ion irradiation reactions (ices were composed of water and varying levels of CO2 and an aliphatic amine)102–105.
Scheme 3.
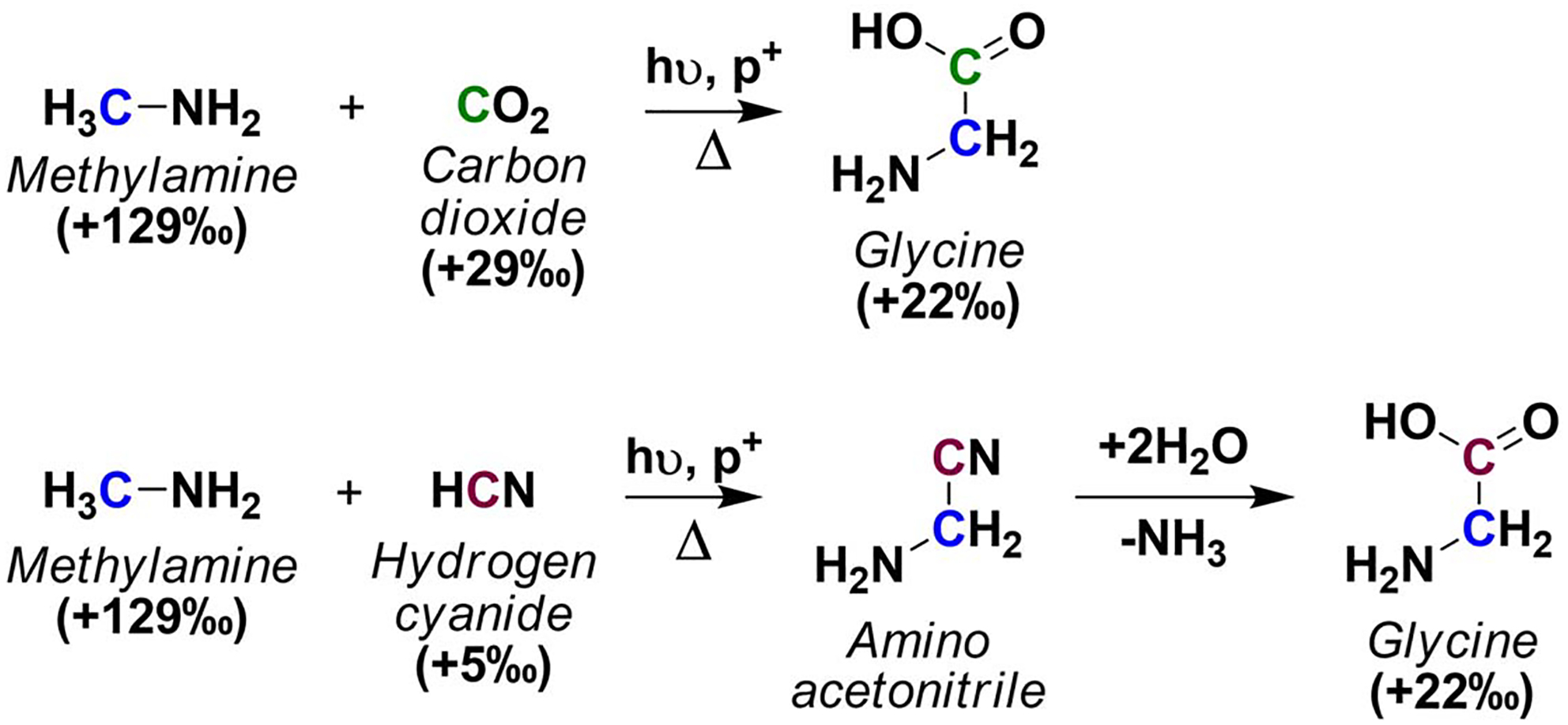
The synthesis of glycine from addition of meteoritic CO2 or HCN to methylamine may occur in photochemical processes, but the likelihood of glycine formation glycine under aqueous hydrothermal conditions remains unknown.
The meteoritic δ13C value of methylamine is higher than that of free CO2, carbonates, and HCN extracted from the Murchison meteorite (Table 1); these measured values, however, are the result of varying levels of processing on an unknown original concentration of molecules. Thus, given the faster reactivity and depletion of 12C-bearing molecules, it may be possible that the δ13C values measured for these materials in meteorites are 13C-enriched relative to their pre-accretionary values. The laboratory δ13C values measured in meteorites are of the products and the remaining unreacted precursors and do not reveal the presolar δ13C values of the reactants.
A close look at the reaction mechanisms involved in the addition of CO2 and HCN to methylamine to synthesize glycine suggest that the likely products of these reactions may not result in the α-amino acid. Unlike photodissociation processes that result in bond cleavage, neutral- radical reactions, and radical-radical recombination, the addition of CO2 to methylamine in aqueous media may only yield methylcarbamic acid (CH3NHCO2H).106,107 Similarly, in the presence of an excess of amines, HCN reacts to form amidines (amines catalyze the polymerization of HCN if present in catalytic concentrations).108,109 However, the synthesis of glycine from the addition of CO2 or HCN to methylamine under meteoritic conditions is yet to be tested; it remains to be seen if in the presence of transition metals or mineral matrixes, carbamates and amidines are useful intermediates in, for example, transition metal-catalyzed reactions for the synthesis of amino acids.
3.3. Synthesis of glycine from NH3 addition to acetic acid.
Similar to the addition of CO2 to methylamine to form glycine, a source of nitrogen such as NH3 may potentially add to acetic acid to produce glycine (Scheme 4). This reaction mechanism, however, has shown mixed results from experiments in the gas-phase;22,23,110 the difficulty in obtaining glycine from these species is based on the acid nature of the carboxyl group and the basic nature of ammonia which result in proton transfer reactions only.110 Therefore, to overcome this physicochemical barrier, Blagojevic et al. (2003) proposed the synthesis of glycine from the reaction of acetic acid with ionized and protonated hydroxylamine (NH2OH+ and NH3OH+ respectively) with successful results.22,23 To the best of our knowledge, this gas-phase reaction has not been tested in ice-irradiated experiments containing acetic acid and hydroxylamine; however, it is very likely that glycine would form from the high energy processes and wide range of reactions mechanisms occurring in irradiated interstellar ice analogs.
Scheme 4.
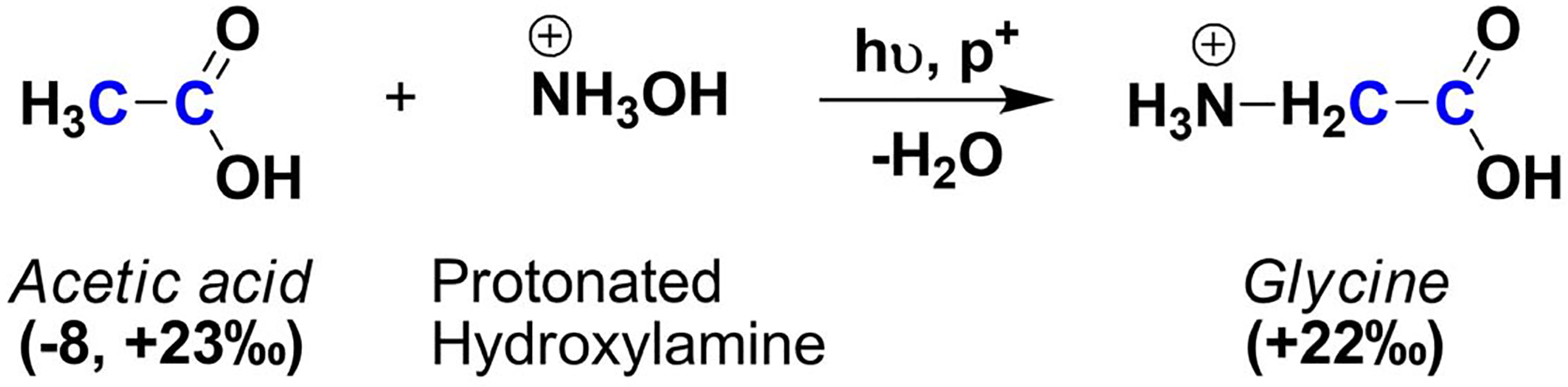
The synthesis of glycine from acetic acid and hydroxylamine may be more challenging inside the asteroid parent body than in interstellar environments.
An astronomical constraint appears from the unsuccessful search for hydroxylamine from interstellar localities;111,112 however, several synthetic pathways for interstellar hydroxylamine have been proposed from various potential interstellar,113–115 including nitric oxide (NO)45,22 which has been detected in the gas phase towards several dark and warm clouds in high concentrations relative to molecular hydrogen.116–118 Furthermore, the theoretical stability of ionized and protonated hydroxyl amine in the ISM suggested that this compound would not react with molecular hydrogen, and thus may be available for the synthesis of larger molecular species such as amino acids.119
Hydroxylamine in any of its forms has not been isolated nor identified from carbonaceous chondrites. However, this inorganic compound may be either present as its ammonia oxide isomer (NH3O) or decomposed into nitrogen oxide species (water, ammonia, and hydrogen) as a result of catalytic effects of transition metals in water and thermal processing inside parent bodies.120–122 The meteoritic δ13C ratios of acetic acid have been measured twice from two different pieces of the Murchison meteorite showing contrasting values (Table 1);35,88 thus, further δ13C analysis of meteoritic acetic acid are needed in order to understand its potential meteoritic linkages to other organic species. The synthesis of glycine from acetic acid and hydroxylamine (either on its ionized or protonated form) is yet to be tested under meteoritic conditions; however, in the absence of highly energetic processes like those resulting in molecular photodissociation, it may prove challenging to propose the addition of hydroxylamine or any of its species to aliphatic moiety (sp3 carbon) such as that in acetic acid, and to avoid side reactions such as proton transfer with the carbonyl group. Indeed, the more likely product may be acetamide formed from the dehydration of ammonium acetate.
3.4. Synthesis of glycine and methylamine from HCN and acetonitrile.
Scheme 5 shows the synthesis of glycine and methylamine using HCN as starting material. HCN has been proposed as a key starting material in interstellar ice chemistry for amino acids synthesis,24,27 the synthesis of glycine from isotopically labeled methanol and HCN in UV-irradiated ices suggested that only low amounts of glycine may form from the oxidation of methanol to formaldehyde in Strecker-type synthesis, and that the majority of glycine (~60%) will preferentially form from HCN when present.27 Similarly, HCN may also be an important precursor of methylamine; Theulé et al. (2011)30 and Kim and Kaiser (2011)31 demonstrated the experimental hydrogenation of HCN through the formation of methanimine as a stable intermediate to yield methylamine in ice-irradiated interstellar analogs.
Scheme 5.
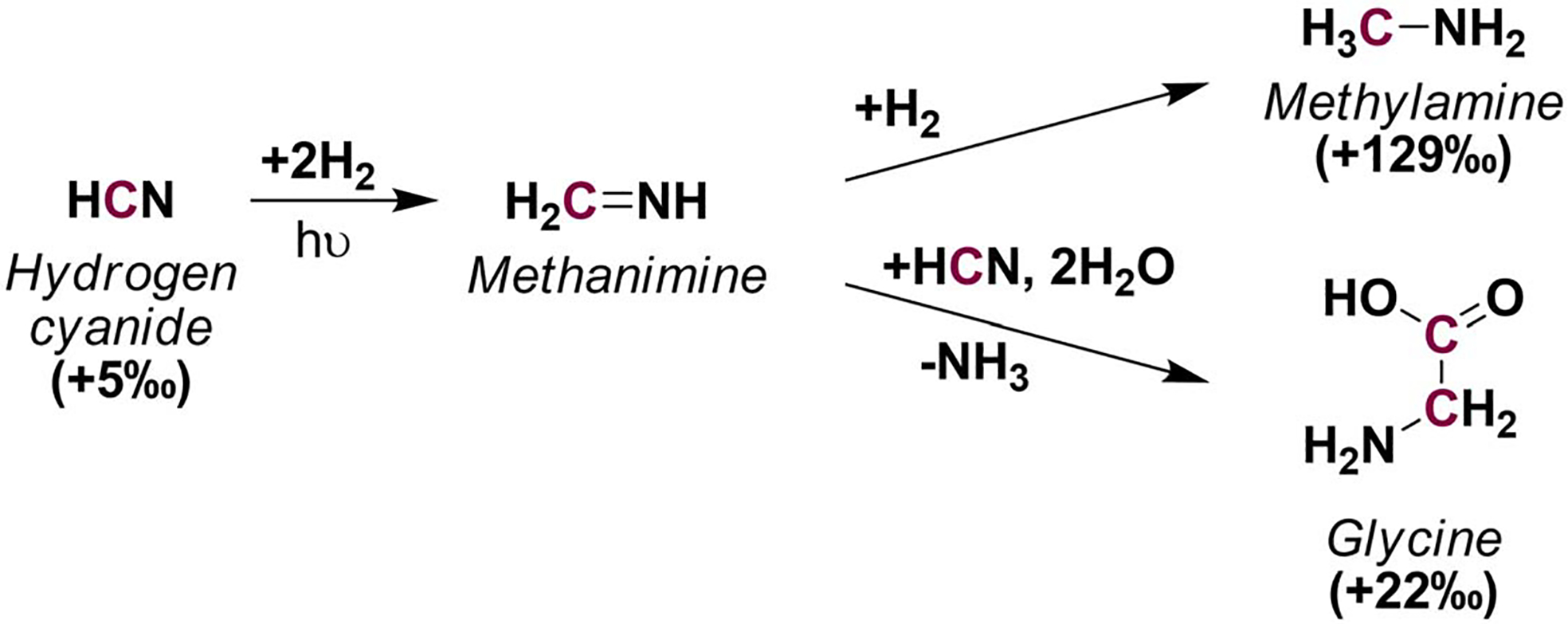
The hydrogenation of HCN has been tested through irradiation of interstellar-ice analogs. This synthetic model however, may need to be tested under parent body conditions to be considered as a plausible synthetic route for meteoritic glycine and methylamine.
Amino acids may also be generated from alkylnitriles; Hudson et al. (2008) proposed the synthesis of amino acids from a proton-irradiated interstellar ice analog composed of acetonitrile and water only. This experiment suggested that the decomposition of acetonitrile would generate HCN and other radical species which could serve as starting materials for the amino acids observed.123 Danger et al. (2011a,b) demonstrated the potential interconversion and synthesis of various species including acetonitrile, α-aminoacetonitrile (a glycine precursor through acid hydrolysis), HCN, and methanimine through thermal activation and VUV irradiation respectively;95,124 suggesting that HCN and acetonitrile may be relevant species for the origins of precursor molecules of glycine and methylamine prior to the formation of the parent body. Although acetonitrile has been observed in interstellar environments,125,126 and could have therefore been incorporated into asteroids, no aliphatic nitrile has yet been extracted and identified from carbonaceous chondrites.
The measured δ13C value of HCN from Murchison is 13C-depleted relative to those found for CO2, carbonates, methylamine, and glycine from the same meteorite (Table 1). As discussed before, these isotopic differences may not be representative of their composition upon accretion into the parent body; however, the δ13C composition of HCN in Murchison may suggest it formed from the 13C-depleted interstellar carbon pool. The isolation of HCN from the Murchison meteorite argues against its total decomposition through polymerization or oxidation, however, the hydrogenation of HCN under parent body conditions to form methanimine would need to be tested in order to consider the origins of glycine and methylamine from this species. Additionally, it would be worth investigating the kinetic isotope effect of the hydrogenation of H12CN and H13CN, either through theoretical calculations or laboratory experiments in order to understand the expected isotopic relationship between HCN and its hydrogenation products. Along those lines, Huang and Sachtler (2000) found a strong D/H isotopic effect during hydrogenation of acetonitrile and deuterated acetonitrile (CH3CN and CD3CN respectively), showing that deuterium atoms add preferentially to the carbon atom, while hydrogen atoms added more selectively to the nitrogen atom.127 The isolation of and δ13C analysis of alkylnitriles is also necessary to fully understand these potential meteoritic connections; however, if the decomposition of these organic species is required to form the observed meteoritic glycine and methylamine, it is challenging to propose a scenario inside the parent body in which these decomposition products would not be quenched by water or other parent body constituents.
4. Synergistic Approaches
In order to thoroughly understand and constrain the various chemical routes associated with the formation of meteoritic glycine and methylamine to a common set of molecular precursors, a multipronged approach that combines astronomical searches, theoretical modeling, and experimental work on reactions in the gas-phase and in interstellar ice analogs, together with the isolation and isotopic analysis of meteoritic species is needed. Organics that formed in the gas phase or in icy grains and their volatile precursors may have been incorporated into the protosolar nebula and later into solar bodies where synthetic and destructive processes also occurred. However, a potential linkage between the formation mechanisms of glycine and methylamine inside interstellar locations, their predicted isotopic outcomes, and their isotopic values registered from meteoritic analyses may provide novel constraints for the likelihood of one synthetic route over the other as summarized in Table 1.
All the molecules serving as potential starting materials for the synthesis of glycine and methylamine discussed here, i.e., CO, HCN, CO2, acetic acid, formaldehyde, methanol, methanimine, and acetonitrile have been detected in various interstellar regions. Further work needs to be done focused on measuring the isotopic composition of these species and in the identification of ever more complex organics in the interstellar medium. More accurate isotope measurement in comets and protoplanetary disks will also help constrain the origins of complex organics by determining the original isotope ratios in each species. These ambitious tasks, however, may remain as pending challenges for a new generation of telescopes like The Atacama Large Millimeter/submillimeter Array (ALMA).
While none of the discussed synthetic routes may be entirely discarded for the synthesis of glycine and methylamine until tested under simulated parent body conditions, the Strecker-cyanohydrin pathway (Scheme 2) is the only synthetic route that is compatible and complementary with the current knowledge of both interstellar processes and the meteoritic analyses. Addition of CO2 (Scheme 3) to methylamine may result in the formation of methylcarbamic acid, while the addition of HCN (Scheme 3) may confer an alternative route but may result in the synthesis of amidines. The occurrence of methylcarbamic acid and amidines, however, may be necessary for other catalytic processes that increase the overall molecular diversity inside the parent body. Similarly, the addition of hydroxylamine to acetic acid to yield glycine (Scheme 4) seems unlikely given the high potential for proton transfer reactions. Indeed, both the addition of CO2 to methylamine and the addition of hydroxylamine to acetic acid would need to occur through breaking a C-H sp3 bond (present in methylamine or acetic acid), a process which may require high energetic input or highly reactive species such as radicals. Amino acids and amines may be generated from the formation of methanimine upon reduction or hydrogenation of HCN (Scheme 5); however, these processes may entirely depend on the nature of the reducing environment. Finally, acetonitrile may decompose to yield cyanide ion (CN−), which may follow the same pathway as HCN discussed above.
Laboratory experiments simulating reactions occurring in the gas-phase and at the surface of interstellar ice particles provide powerful insights into the nature of the chemical processes inside cold molecular clouds and star forming regions. We now understand that that a plethora of complex organic molecules may result from the interaction of ionizing radiation (cosmic rays, UV photons, soft X-rays, etc.) and a mixture of volatile hydrogen, carbon, nitrogen and oxygen sources (H2O, CO, CH3OH, HCN, NH3, etc.) in presolar and interstellar environments. However, greater effort should now be placed on quantitative analysis focused on measuring reaction rates, as well as the inherent molecular stability, abundance, isomeric distribution, and isotope effect of organic products.
In addition, relatively little attention has been paid to the identification of these volatile species from meteoritic sources and even fewer studies have been reported of their meteoritic isotopic values. Several families of complex organic molecules such as PAHs, amino acids, amines and carboxylic acid have been identified and isotopically characterized from carbonaceous chondrites,10,88,89,128 however, much less is known about the abundance and isotopic distributions of free CO, CO2, HCN and ammonia (Yuen et al. 1984; Pizzarello et al. 2011; Pizzarello and Williams 2012; Pizzarello 2014).35,58,93,94 Furthermore, the Murchison meteorite has been found to contain a heterogeneous composition, meaning that different pieces analyzed may contain similar but not the same molecular and isotopic distributions;89,129 therefore, it remains imperative to expand the stable isotopic studies of CO, HCN, CO2, and acetic acid, as these measurements have only been performed on one meteorite for each compound. In addition, there are meteoritic organic compounds such as alcohols, aldehydes, and ketones (e.g. formaldehyde and methanol) which have been identified and quantified in Murchison and other meteorites, but without measurements of their corresponding isotopic values, and there are other plausible intermediates such as methanimine, alkylnitriles, aminonitriles, and hydroxynitrile that have not been searched for from any meteorite. Without a thorough assessment of the abundance and isotopic composition of these species, it remains difficult to investigate their potential synthetic relationship with more complex compounds. These should be high priority targets for study in meteorites as well as the large sample to be returned from asteroid Ryugu by JAXA’s Hayabusa2 sample return mission in 2020 and from asteroid Bennu by NASA’s OSIRIS-REx sample return mission in 2023.
5. Conclusion
Meteoritic glycine and methylamine may have formed in pre-solar environments and/or inside meteorite parent bodies. Separating synthetic processes that may have occurred before the formation of the parent body from those that occurred through aqueous and thermal processes inside the parent body is challenging. Even more challenging is assessing the synthesis/destruction of an original compound pool and the level of 13C-fractionation experienced over different periods of time and physicochemical conditions. Therefore, we face two main challenges which may be solved through a future systematic quantification and experimental modeling. Evaluating the effects of aqueous and thermal processing on the abundance and isotopic compositions of meteoritic organic compounds may lead to a more comprehensive evaluation of the origins of meteoritic organic compounds. Estimating the magnitude of fractionation expected during synthesis and parent body processing remains highly challenging and much work is needed to fully understand the kinetics and reaction efficiencies, as well as the pre-parent body molecular abundances and isotopic compositions of these molecules.
We have evaluated the potential synthetic relationships between glycine and methylamine using their isotopic compositions and those of their potential precursor molecules from the Murchison meteorite. Our analysis aimed to evaluate various meteoritic species as probes into parent body chemistry and to link proposed formation mechanisms with data collected through astronomical observations, experiments in both the gas-phase and in irradiated ice interstellar analogs, and theoretical modeling. However, the main conclusion of this exercise is that there is still a large number of meteoritic and laboratory analyses, as well as telescopic observations, and theoretical modeling that must be performed before there is sufficient data to fully understand the synthesis of extraterrestrial organic compounds present in meteorites. Ideally, we would be able to draw phase diagrams for the synthesis of glycine and methylamine with respect to variables such as parent body temperatures and concentration of reactants for example; unfortunately, the original molecular concentrations and isotopic compositions of the precursor molecules remain unknown. Similarly we have only recently started to unveil the varying levels of aqueous and thermal effects occurred in different parent bodies, albeit, the magnitude of this processing on the organic composition of carbonaceous chondrites is still poorly understood, at least from a mechanistic standpoint. Therefore, future interdisciplinary efforts are needed to further our understanding of these and other meteoritic organic compounds.
Table 2.
Interstellar analogous synthesis of glycine and methylamine and their potential implications for their synthesis inside the parent body.
| Synthesis from/by | Gly/methylamine seen in gas-phase reactions? | Gly/methylamine seen in irradiated ice analogs? | Possibility of reaction inside the asteroid parent body |
|---|---|---|---|
| CO and/or formaldehyde (Scheme 2) | n.d. | Yesa,24–27 | Glycine may form through the Strecker synthesis |
| CO2 addition to methylamine (Scheme 3) | Noa | Yes101,102 | May produce methylcarbamic acid instead of glycine |
| HCN addition to methylamine (Scheme 3) | n.d. | n.d.* | May yield amidines or polymerization of HCN depending on abundances instead of glycine |
| NH3/hydroxylamine addition to acetic acid (Scheme 4) | No109/Yes22,23 | n.d.* | May result in proton transfer reactions instead |
| HCN hydrogenation (Scheme 5) | n.d. | Yes30,31 | Would depend on the reduction potential of the molecular environment |
n.d: Experiment has not been performed or reported in the literature.
While ice analogs are usually a combination of CO, CO2, methanol, HCN, and ammonia in water, it has been suggested that at least a portion of glycine and methylamine would form from the hydrogenation of CO to formaldehyde and subsequent Strecker reaction and nucleophilic substitution respectively (Scheme 2)24–27.
This experiment may have not been performed or reported in the literature; however, it is very likely that glycine and methylamine will form from the molecular photodissociation of acetic acid and hydroxylamine to yield highly reactive radical/ionic species inside ice irradiated experiments.
ACKNOWLEDGEMENTS
This research was supported by the NASA Astrobiology Institute and the Goddard Center for Astrobiology, and a grant from the Simons Foundation (SCOL award 302497 to J.P.D.).
REFERENCES
- 1.Oró J Comets and the formation of biochemical compounds on the primitive Earth. Nature 1961, 190, 389–390. [DOI] [PubMed] [Google Scholar]
- 2.Anders E Pre-biotic organic matter from comets and asteroids. Nature 1989, 342, 255–257. [DOI] [PubMed] [Google Scholar]
- 3.Chyba C; Sagan C Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 1992, 355, 125–132. [DOI] [PubMed] [Google Scholar]
- 4.Botta O; Glavin DP; Kminek G; Bada JL Relative amino acid concentrations as a signature for parent body processes of carbonaceous chondrites. Orig. Life Evol. Biosph 2002, 32, 143–163. [DOI] [PubMed] [Google Scholar]
- 5.Burton AS; Stern JC; Elsila JE; Glavin DP; Dworkin JP Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev 2012, 41, 5459–5472. [DOI] [PubMed] [Google Scholar]
- 6.Cobb AK; Pudritz RE Nature’s starships. I. Observed abundances and relative frequencies of amino acids in meteorites. Astrophys. J 2014, 783, 140 (12pp). [Google Scholar]
- 7.Elsila JE; Aponte JC; Blackmond DG; Burton AS; Dworkin JP; Glavin DP Meteoritic amino acids: Diversity in compositions reflects parent body histories. ACS Cent. Sci 2016, 2, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aponte JC; Dworkin JP; Elsila JE Assessing the origins of aliphatic amines in the Murchison meteorite from their compound-specific carbon isotopic ratios and enantiomeric composition. Geochim. Cosmochim. Acta 2014, 141, 331–345. [Google Scholar]
- 9.Aponte JC; Dworkin JP; Elsila JE Indigenous aliphatic amines in the aqueously altered Orgueil meteorite. Meteorit. Planet. Sci 2015, 50, 1733–1749. [Google Scholar]
- 10.Aponte JC; McLain HL; Dworkin JP; Elsila JE Aliphatic Amines in Antarctic CR2, CM2 and CM1/2 Carbonaceous Chondrites. Geochim. Cosmochim. Acta 2016, 189, 296–311. [Google Scholar]
- 11.Pizzarello S; Yarnes T Enantiomeric excesses of chiral amines in ammonia-rich carbonaceous meteorites. Earth Planet. Sci. Lett 2016, 443, 176–184. [Google Scholar]
- 12.Jungclaus GA; Cronin JR; Moore CB; Yuen GU Aliphatic amines in the Murchison meteorite. Nature 1976, 261, 126–128. [Google Scholar]
- 13.Pizzarello S; Feng X; Epstein S; Cronin JR Isotopic analyses of nitrogenous compounds from the Murchison meteorite: ammonia, amines, amino acids, and polar hydrocarbons. Geochim. Cosmochim. Acta 1994, 58, 5579–5587. [DOI] [PubMed] [Google Scholar]
- 14.Pizzarello S; Holmes W Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar]
- 15.Sandford SA; Aléon J; Alexander CMO’D;Araki T; Bajt S; Baratta GA; Borg J; Brucato JR; Burchell MJ; Busemann H; Butterworth A; Clemett SJ; Cody G; Colangeli L; Cooper G; D’Hendecourt L; Djouadi Z; Dworkin JP; Ferrini G; Fleckenstein H; Flynn GJ; Franchi IA; Fries M; Gilles MK; Glavin DP; Gounelle M; Grossemy F; Jacobsen C; Keller LP; Kilcoyne D; Leitner J; Matrajt G; Meibom A; Mennella V; Mostefaoui S; Nittler LR; Palumbo ME; Robert F; Rotundi A; Snead CJ; Spender MK; Steele A; Stephan T; Tyliszczak T; Westphal AJ; Wirick S; Wopenka B; Yabuta H; Zare RN; Zolensky M Organics captured from comet Wild 2 by the Stardust spacecraft. Science 2006, 314, 1720–1724. [DOI] [PubMed] [Google Scholar]
- 16.Glavin DP; Dworkin JP; Sandford SA Detection of cometary amines in samples returned by Stardust. Meteorit. Planet. Sci 2008, 43, 399–413. [Google Scholar]
- 17.Elsila JE; Glavin DP; Dworkin JP Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci 2009, 44, 1323–1330. [Google Scholar]
- 18.Altwegg K; Balsiger H; Bar-Nun A; Berthelier J-J; Bieler A; Bochsler P; Briois C; Calmonte U; Combi MR; Cottin H; De Keyser J; Dhooghe F; Fiethe B; Fuselier SA; Gasc S; Gombosi TI; Hansen KC; Haessig M; Jäckel A; Kopp E; Korth A; Le Roy L; Mall U; Marty B; Mousis O; Owen T; Rème H; Rubin M; Sémon T; Tzou C-Y; Waite JH; Wurz P Prebiotic chemicals -amino acid and phosphorus- in the coma of comet 67P/Churyumov-Gerasimenko. Sci. Adv 2016, 2, e1600285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goesmann F; Rosenbauer H; Bredehöft JH; Cabane M; Ehrenfreund P; Gautier T; Giri C; Krüger H; Le Roy L; MacDermott AJ; McKenna-Lawlor S; Meierhenrich UJ; Muñoz-Caro GM; Raulin F; Roll R; Steele A; Steininger H; Sternberg R; Szopa C; Thiemann W; Ulamec S Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349, aab0689. [DOI] [PubMed] [Google Scholar]
- 20.Gardner EP; McNesby JR Methylamine formation in the vacuum ultraviolet photolysis of methane and ammonia mixtures. J. Photochem 1980, 113, 353–356. [Google Scholar]
- 21.Ogura K; Migita CT; Yamada T Photochemical formation of methylamine and ethylenediamine from gas mixtures of methane, ammonia, and water. Chem. Lett 1988, 1563–1566. [Google Scholar]
- 22.Blagojevic V; Petrie S; Bohme DK Gas-phase syntheses for interstellar carboxylic and amino acids. Mon. Not. Astron. Soc 2003, 339, L7–L11. [Google Scholar]
- 23.Snow JL; Orlova G; Blagojevic V; Bohme DK Gas-phase ionic syntheses of amino acids: β versus α. J. Am. Chem. Soc 2007, 129, 9910–9917. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein MP; Dworkin JP; Sandford SA; Cooper GW; Allamandola LJ Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Caro GM; Meierhenrich UJ; Schutte WA; Barbier B; Segovia AA; Rosenbauer H; Thiemann WH-P; Brack A; Greenberg JM Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [DOI] [PubMed] [Google Scholar]
- 26.Nuevo M; Meierhenrich UJ; Muñoz-Caro GM; Dartois E; D’Hendecourt L; Deboffle D; Auger G; Blanot D; Bredehöft J-H; Nahon L The effects of circularly polarized light on amino acid enantiomers produced by the UV irradiation of interstellar ice analogs. Astron. Astrophys 2006, 457, 741–751. [Google Scholar]
- 27.Elsila JE; Dworkin JP; Bernstein MP; Martin MP; Sandford SA Mechanisms of amino acid formation in interstellar ice analogs. Astrophys. J 2007, 660, 911–918. [Google Scholar]
- 28.Takano Y; Takahashi J; Kaneko T; Marumo K; Kobayashi K Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet Sci. Lett 2007, 254, 106–114. [Google Scholar]
- 29.De Marcellus P; Meinert C; Nuevo M; Filippi J-J; Danger G; Deboffle D; Nahon L; Le S. d’Hendecourt L; Meierhenrich UJ Non-racemic amino acid production by ultraviolet irradiation of achiral interstellar ice analogs with circularly polarized light. Astrophys. J. Lett 2011, 727, L27 (6pp). [Google Scholar]
- 30.Theulé P; Borget F; Mispelaer F; Danger G; Duvernay F; Guillemin JC; Chiavassa T Hydrogenation of solid hydrogen cyanide HCN and methanimine CH2NH at low temperature. Astron. Astrophysics 2011, 534, A64 (6pp). [Google Scholar]
- 31.Kim YS; Kaiser RI On the formation of amines (RNH2) and the cyanide anion (CN−) in electron-irradiated ammonia-hydrocarbon interstellar model ices. Astrophys. J 2011, 829, 68 (7pp). [Google Scholar]
- 32.Modica P; Meinert C; De Marcellus P; Nahon L; Meierhenrich UJ; Le S. d’Hendecourt L Enantiomeric excesses induced in amino acids by ultraviolet circularly polarized light irradiation of extraterrestrial ice analogs: A possible source of asymmetry for prebiotic chemistry. Astrophys. J. Lett 2014, 788, 79 (11pp). [Google Scholar]
- 33.Miller SL A production of amino acids under possible primitive Earth conditions. Science 1953, 117, 528–529. [DOI] [PubMed] [Google Scholar]
- 34.Parker ET ; Cleaves HJ; Dworkin JP; Glavin DP ; Callahan M; Aubrey A; Lazcano A; Bada JL Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc. Natl. Acad. Sci. U.S.A 2011, 108, 5526–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen G; Blair N; Des Marais DJ; Chang S Carbon isotopic composition of individual, low molecular weight hydrocarbons and monocarboxylic acids from the Murchison meteorite. Nature 1984, 307, 252–254. [DOI] [PubMed] [Google Scholar]
- 36.Sandford SA; Bernstein MP; Dworkin JP Assessment of the interstellar processes leading to deuterium enrichment in meteoritic organics. Meteor. Planet. Sci 2001, 36, 1117–1133. [Google Scholar]
- 37.Robert F The D/H ratio in chondrites. Space Sci. Rev 2003, 106, 87–101. [Google Scholar]
- 38.Le Guillou C; Bernard S; Brearley AJ; Remusat L Evolution of organic matter in Orgueil, Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochim. Cosmochim. Acta 2014, 131, 368–392. [Google Scholar]
- 39.Beck P; Garenne A; Quirico E; Bonal L; Montes-Hernandez G; Moynier F; Schmitt B Transmission infrared spectra (2–25 μm) of carbonaceous chondrites (CI, CM, CV-CK, CR, C2 ungrouped): Mineralogy, water, and asteroidal processes. Icarus 2014, 229, 263–277. [Google Scholar]
- 40.McAdam MM; Sunshine JM; Howard KT; McCoy TM Aqueous alteration on asteroids: Linking the mineralogy and spectroscopy of CM and CI chondrites. Icarus 2015, 245, 320–332. [Google Scholar]
- 41.Kaluna HM; Masiero JR; Meech KJ Space weathering trends among carbonaceous asteroids. Icarus 2016, 264, 62–71 [Google Scholar]
- 42.Tielens AGGM; Hagen W Model calculations of the molecular composition of interstellar grain mantles. Astron. Astrophys 1982, 114, 245–260. [Google Scholar]
- 43.Charnley SB On the Nature of Interstellar Organic Chemistry, in Astronomical and Biochemical Origins and the Search for Life in the Universe, eds. Cosmovici CB, Bowyer S & Werthimer D, Editrice Compositori, Bologna, p. 89, 1997. [Google Scholar]
- 44.Ehrenfreund P; Charnley SB Organic molecules in the interstellar medium, comets, and meteorites: A voyage from dark clouds to the early Earth. Annu. Rev. Astron. Astrophys 2000, 38, 427–483. [Google Scholar]
- 45.Charnley SB The Bridge between the Big Bang and Biology, ed. Giovannelli F, Consiglio Nazionale delle Ricerche, Italy, p. 139, 2001. [Google Scholar]
- 46.Charnley SB; Rodgers SD Clouds, clumps, cores and comets- a cosmic chemical connection? Adv. Geosci 2009, 15, 211. [Google Scholar]
- 47.Taquet V; Wirström E; Charnley SB Formation and recondensation of complex organic molecules during protostellar luminosity outbursts. Astrophys. J 2016, 821, 46 (12pp). [Google Scholar]
- 48.Ziurys LM The chemistry in circumstellar envelopes of evolved stars: Following the origin of the elements to the origin of life. Proc. Natl. Acad. Sci. U.S.A 2006, 103, 12274–12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbst E; van Dishoeck EF Complex organic interstellar molecules. Annu. Rev. Astro. Astrophys 2009, 47, 427–480. [Google Scholar]
- 50.Öberg KI; Fayolle EC; Reiter JB; Cyganowski C Complex molecule formation around massive young stellar objects. Farad. Discuss 2014, 168, 81–101. [DOI] [PubMed] [Google Scholar]
- 51.Turner BE Detection of doubly deuterated interstellar formaldehyde (D2CO) - an indicator of active grain surface chemistry. Astrophys. J. Lett 1990, 362, L29–L33. [Google Scholar]
- 52.Latter WB; Walker CK; Maloney PR Detection of the carbon monoxide ion (CO+) in the interstellar medium and a planetary nebula. Astrophys. J 1993, 419, L97–L100. [Google Scholar]
- 53.Ohishi M; Ishikawa S-I; Amano T; Oka H; Irvine WM; Dickens JE Detection of a new interstellar molecular ion, H2COH+ (protonated formaldehyde). Astrophys. J. Lett 1996, 471, L61–L64. [DOI] [PubMed] [Google Scholar]
- 54.Bacmann A; García-García E; Faure A Detection of protonated formaldehyde in the prestellar core L1689B. Astron. Astrophys 2016, 588, L8. [Google Scholar]
- 55.Jehin E; Manfroid J; Hutsemékers D; Arpigny C; Zucconi J-M Isotopic ratios in comets: Status and perspectives. Earth Moon Planet 2009, 105, 167–180. [Google Scholar]
- 56.Mumma MJ; Charnley SB The chemical composition of comets-emerging taxonomies and natal heritage. Annu. Rev. Astron. Astrophys 2011, 49, 471–524. [Google Scholar]
- 57.A’Hearn MF; M. Feaga L; Keller HU; Kawakita H; Hampton DL; Kissel J; Klaasen KP; McFadden LA; Meech KJ; Schultz PH; Sunshine JM; Thomas PC; Veverka J; Yeomans DK; Besse S; Bodewits D; Farnham TL; Groussin O; Kelley MS; Lisse CM; Merlin F; Protopapa S; Wellnitz DD Cometary volatiles and the origin of comets. Astrophys. J 2012, 758, 29 (8pp). [Google Scholar]
- 58.Pizzarello S The nitrogen isotopic composition of meteoritic HCN. Astrophys. J. Lett 2014, 796, L25 (4pp). [Google Scholar]
- 59.Kuan Y-J; Charnley SB; Huang H-C; Tseng W-L; Kisiel Z Interstellar glycine. Astrophys. J 2003, 593, 848–867. [Google Scholar]
- 60.Ceccarelli C; Loinard L; Castets A; Faure A; Lefloch B Search for glycine in the solar type protostar IRAS 16293–2422. Astron. Astrophys 2000, 362, 1122–1126. [Google Scholar]
- 61.Snyder LE; Lovas FJ; Hollis JM; Friedel DN; Jewell PR; Remijan A; Ilyushin VV; Alekseev EA; Dyubko SF A rigorous attempt to verify interstellar glycine. Astrophys. J 2005, 619, 914–930. [Google Scholar]
- 62.Woon DE Pathways to glycine and other amino acids in ultraviolet-irradiated astrophysical ices determined via chemical modeling. Astrophys. J 2002, 571, L177–L180. [Google Scholar]
- 63.Largo L; Redondo P; Rayón VM; Largo A; Barrientos C The reaction between NH3+ and CH3COOH: A possible process for the formation of glycine precursors in the interstellar medium. Astron. Astrophys 2010, 516, A79–A86. [Google Scholar]
- 64.Pilling S; Baptista L; Boechat-Roberty HM; Andrade DPP Formation routes of interstellar glycine involving carboxylic acids: Possible favoritism between gas and solid phase Astrobiology 2011, 11, 883–893. [DOI] [PubMed] [Google Scholar]
- 65.Nhlabatsi ZP; Bhasi P; Sitha S Possible interstellar formation of glycine from the reaction of CH2=NH, CO and H2O: Catalysis by extra water molecules through the hydrogen relay transport. Phys. Chem. Chem. Phys 2016, 18, 375–381. [DOI] [PubMed] [Google Scholar]
- 66.Kaifu N; Morimoto M; Nagane K; Akabane K; Iguchi T; Takagi K Detection of interstellar methylamine. Astrophys. J 1974, 191, L135–L137. [Google Scholar]
- 67.Fourikis N; Takagi K; Saito S Probable detection of interstellar methylamine-D (CH3NHD). Astrophys. J 1977, 212, L33–L37. [Google Scholar]
- 68.Dickens JE; Irvine WM; DeVries CH; Ohishi M Hydrogenation of interstellar molecules: A survey for methylenimine (CH2NH). Astrophys. J 1997, 479, 307–312. [DOI] [PubMed] [Google Scholar]
- 69.Halfen DT; Ilyushin VV; Ziurys LM Insights into surface hydrogenation in the interstellar medium: Observations of methanimine and methyl amine in Sgr B2(N). The Astrophys. J 2013, 797, 66 (11pp). [Google Scholar]
- 70.Suzuki T; Ohishi M; Hirota T; Saito M; Majumdar L; Wakelam V Survey observations of a possible glycine precursor, methanimine (CH2NH). Astrophys. J 2016, 825, 79 (14pp). [Google Scholar]
- 71.Smith RL; Pontoppidan KM; Young ED; Morris MR. Heterogeneity in 12CO/13CO abundance ratios toward solar-type young stellar objects. Astrophys. J 2015, 813, 120 (16pp). [Google Scholar]
- 72.Scott PC; Asplund M; Grevesse N; Sauval AJ Line formation in solar granulation. VII. CO lines and the solar C and O isotopic abundances. Astron. Astrophys 2006, 456, 675–688. [Google Scholar]
- 73.Qi C; D’Alessio P; Öberg KI; Wilner DJ; Hughes AM; Andrews SM; Ayala S Resolving the CO snow line in the disk around HD 163296. Astrophys. J 2011, 740, 84 (18pp). [Google Scholar]
- 74.Woods PM; Willacy K Carbon isotope fractionation in protoplanetary disks. Astrophys. J 2009, 693, 1360–1378. [Google Scholar]
- 75.Langer WD; Graedel TE; Frerking MA; Armentrout PB Carbon and oxygen isotope fractionation in dense interstellar clouds. Astrophys. J 1984, 277, 581–604. [Google Scholar]
- 76.Sakai N; Saruwatari O; Sakai T; Takano S; Yamamoto S Abundance anomaly of the 13C species of CCH. Astron. Astrophys 2010, 512, A31 (10pp). [Google Scholar]
- 77.Yoshida K; Sakai N; Tokudome T; López-Sepulcre A; Watanabe Y; Takano S; Lefloch B; Ceccarelli C; Bachiller R; Caux E; Vastel C; Yamamoto S Abundance anomaly of the 13C isotopic species of c-C3H2 in the low-mass star formation region L1527. Astrophys. J 2015, 807, 66 (9pp). [Google Scholar]
- 78.Wirström ES; Charnley SB; Geppert WD; Persson CM Observations of carbon isotopic fractionation in interstellar formaldehyde (abstract #1161). 43rd Lunar and Planetary Science Conference 2012, CD-ROM. [Google Scholar]
- 79.Milam SN; Savage C; Brewster MA; Ziurys LM; Wyckoff S The 12C/13C isotope gradient derived from millimeter transitions of CN: The case for galactic chemical evolution. Astrophys. J 2005, 634, 1126–1132. [Google Scholar]
- 80.Des Marais DJ; Donchin JH; Nehring NL; Truesdell AH Molecular carbon isotopic evidence for the origin of geothermal hydrocarbons. Nature 1981, 292, 826–828. [Google Scholar]
- 81.Chang S; Des Marais DJ; Mack R; Miller SL; Strathearn G Earth’s Earliest Biosphere: Its Origin and Early Evolution. ed. Schopf JW. Princeton University Press, 1983. [Google Scholar]
- 82.Grady MM; Wright IP; Swart PK; Pillinger CT The carbon and oxygen isotopic composition of meteoritic carbonates. Geochim. Cosmochim. Acta 1988, 52, 2855–2866. [Google Scholar]
- 83.Cody GD; Alexander CM O’D. NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrite groups. Geochim. Cosmochim. Acta 2005, 69, 1085–1097. [Google Scholar]
- 84.Alexander MO’D; Bowden R; Fogel ML; Howard KT Carbonate abundances and isotopic compositions in chondrites. Meteorit. Planet. Sci 2015, 50, 523–849. [Google Scholar]
- 85.Ehrenfreund P; Glavin DP; Botta O; Cooper G; Bada JL Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of CI type carbonaceous chondrites. Proc. Natl. Acad. Sci. U.S.A 2001, 98, 2138–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glavin DP; Callahan MP; Dworkin JP; Elsila JE The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci 2010, 45, 1948–1972. [Google Scholar]
- 87.Alexander CM; Bowden R; Fogel ML; Howard KT; Herd CDK; Nittler LR The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 2012, 337, 721–723. [DOI] [PubMed] [Google Scholar]
- 88.Huang Y; Wang Y; Alexandre MR; Lee T; Rose-Petruck C; Fuller M; Pizzarello S Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim. Cosmochim. Acta 2005, 69, 1073–1084. [Google Scholar]
- 89.Elsila JE; Charnley SB; Burton AS; Glavin DP; Dworkin JP Compound-specific carbon, nitrogen, and hydrogen isotopic ratios for amino acids in CM and CR chondrites and their use in evaluating potential formation pathways. Meteor. Planet. Sci 2012, 47, 1517–1536. [Google Scholar]
- 90.Engel MH; Macko SA; Silfer JA Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature 1990, 348, 47–49. [DOI] [PubMed] [Google Scholar]
- 91.Pizzarello S; Huang Y; Fuller M The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 2004, 68, 4963–4969. [Google Scholar]
- 92.Martins Z; Alexander CMO’D; Orzechowska GE; Fogel ML; Ehrenfreund P Indigenous amino acids in primitive CR meteorites. Meteorit. Planet. Sci 2007, 42, 2125–2136. [Google Scholar]
- 93.Pizzarello S; Williams LB Ammonia in the early Solar System: An account from carbonaceous meteorites. Astrophys. J 2012, 749, 161 (6pp). [Google Scholar]
- 94.Pizzarello S; Williams LB; Lehman J; Holland GP; Yarger JL Abundant ammonia in primitive asteroids and the case for a possible exobiology. Proc. Natl. Acad. Sci. USA 2011, 108, 4303–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Danger G; Borget F; Chomat M; Duvernay F; Theulé P; Guillemin J-C; d’Hendecourt LLS; Chiavassa T Experimental investigation of aminoacetonitrile formation through the Strecker synthesis in astrophysical-like conditions: Reactivity of methanimine (CH2NH), ammonia (NH3), and hydrogen cyanide (HCN). Astron. Astrophys 2011a, 535, A47 (9pp). [Google Scholar]
- 96.Peltzer ET; Bada JL; Schlesinger G; Miller SL The chemical conditions on the parent body of the Murchison meteorite: some conclusions based on amino, hydroxy and dicarboxylic acids. Adv. Space Res 1984, 4, 69–74. [DOI] [PubMed] [Google Scholar]
- 97.Lerner NR; Peterson E; Chang S The Strecker synthesis as a source of amino acids in carbonaceous chondrites-Deuterium retention during synthesis. Geochim. Cosmochim. Acta 1993, 57, 4713–4723. [DOI] [PubMed] [Google Scholar]
- 98.Gunanathan C; Milstein D Selective synthesis of primary amines directly from alcohols and ammonia. Angew. Chem. Int. Ed 2008, 47, 8661–8664. [DOI] [PubMed] [Google Scholar]
- 99.Shimizu K; Kon K; Onodera W; Yamazaki H; Kondo JN Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia. ACS Catal. 2013, 3, 112–117. [Google Scholar]
- 100.Yan T; Feringa BL; Barta K Iron catalysed direct alkylation of amines with alcohols. Nature Commun 2014, 5, 5602. [DOI] [PubMed] [Google Scholar]
- 101.Jackson DM; Stibrich NJ; Adams NG; Babcock LM A selected ion flow tube study of the reactions of a sequence of ions with amines. Int. J. Mass Spectrom 2005, 243, 115–120. [Google Scholar]
- 102.Holtom PD; Bennett CJ; Osamura Y; Mason NJ; Kaiser RI A combined experimental and theoretical study on the formation of the amino acid glycine (NH2CH2COOH) and its isomer (CH3NHCOOH) in extraterrestrial ices. Astrophys. J 2005, 626, 940–952. [Google Scholar]
- 103.Hudson RL; Lewis AS; Moore MH; Dworkin JP; Martin MP Enigmatic isovaline: Investigating the stability, racemization, and formation of a non-biological meteoritic amino acid. Astron. Soc. Pacific Conf. Series 2009, 420,157–162. [Google Scholar]
- 104.Bossa J-B; Duvernay F; Theulé P; Borget F; d’Hendecourt L; Chiavassa T Methylammonium methylcarbamate thermal formation in interstellar Ice analogs: a glycine salt precursor in protostellar environments. Astron. Astrophys 2009, 506, 601–608. [Google Scholar]
- 105.Lee C-W; Kim J-K; Moon E-S; Minh YC; Kang H Formation of glycine on ultraviolet-irradiated interstellar ice-analog films and implications for interstellar amino acids. Astrophys. J 2009, 697, 428–435. [Google Scholar]
- 106.Murphy LJ; Robertson KN; Kemp RA; Tuononen HM; Clyburne JAC Structurally simple complexes of CO2. Chem. Commun 2015, 51, 3942–3956. [DOI] [PubMed] [Google Scholar]
- 107.Mohammed FS; Kitchens CL Reduced reactivity of amines against nucleophilic substitution via reversible reaction with carbon dioxide. Molecules 2016, 21 (11pp). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Erickson JG Reactions of some amines with hydrogen cyanide. J. Org. Chem 1955, 20, 1569–1572. [Google Scholar]
- 109.Wang J; Xu F; Cai T; Shen Q Addition of amines to nitriles catalyzed by ytterbium amides: An efficient one-step synthesis of monosubstituted n-arylamidines. Org. Lett 2008, 10, 445–448. [DOI] [PubMed] [Google Scholar]
- 110.Jackson DM; Stibrich NJ; McLain JL; Fondren LD; Adams NG; Babcock LM A selected ion flow tube study of the reactions of various nitrogen containing ions with formic acid, acetic acid, and methyl formate. Int. J. Mass Spectrom 2005, 247, 55–60. [Google Scholar]
- 111.Pulliam RL; McGuire BA; Remijan AJ A search for hydroxylamine (NH2OH) toward select astronomical sources. Astrophys. J 2012, 751, 1 (7pp). [Google Scholar]
- 112.McGuire BA; Carroll PB; Dollhopf NM; Crockett NR; Corby JF; Loomis RA; Burkhardt AM; Shingledecker C; Blake GA; Remijan AJ CSO and CARMA observations of L1157. I. A deep search for hydroxylamine (NH2OH). Astrophys. J 2015, 812, 76 (9pp). [Google Scholar]
- 113.Nishi N; Shinohara H; Okuyama T Photodetachment, photodissociation, and photochemistry of surface molecules of icy solids containing NH3 and pure H2O ices. J. Chem. Phys 1984, 80, 3898–3910. [Google Scholar]
- 114.Zheng W; Kaiser RI Formation of hydroxylamine (NH2OH) in electron-irradiated ammonia-water ices. J. Phys. Chem. A 2010, 114, 5251–5255. [DOI] [PubMed] [Google Scholar]
- 115.He J; Vidali G; Lemaire J-L; Garrod RT Formation of hydroxylamine on dust grains via ammonia oxidation. Astrophys. J 2015, 799, 49 (9pp). [Google Scholar]
- 116.Liszt HS; Turner BE Microwave detection of NO. Astrophys. J 1978, 224, L73–L76. [Google Scholar]
- 117.McGonagle D; Ziurys LM; Irvine WM; Minh YC Detection of nitric oxide in the dark cloud L134N. Astrophys. J 1990, 359, 121–124. [DOI] [PubMed] [Google Scholar]
- 118.Gerin M; Viala Y; Pauzat F; Ellinger Y The abundance of nitric oxide in molecular clouds. Astron. Astrophys 1992, 266, 463–478. [Google Scholar]
- 119.Largo L; Rayón VM; Barrientos C; Largo A; Redondo P Stability of protonated and ionized hydroxylamine in the interstellar medium. Chem. Phys. Lett 2009, 476, 174–177. [Google Scholar]
- 120.Iwata Y; Koseki H Decomposition of hydroxylamine/water solution with added iron ion. J. Hazard. Mater 2003, 104, 39–49. [DOI] [PubMed] [Google Scholar]
- 121.Wang Q; Wei C; Pérez LM; Rogers WJ; Hall MB; Mannan MS Thermal decomposition pathways of hydroxylamine: Theoretical investigation on the initial steps. J. Phys. Chem. A 2010, 114, 9262–9269. [DOI] [PubMed] [Google Scholar]
- 122.Adamopoulou T; Papadaki MI; Kounalakis M; Vazquez-Carreto V; Pineda-Solano A; Wang Q; Mannan MS J. Hazard. Mater 2013, 254–255, 382–389. [DOI] [PubMed] [Google Scholar]
- 123.Hudson RL; Moore MH; Dworkin JP; Martin MP; Pozun ZD Amino acids from ion-irradiated nitrile-containing ices. Astrobiology 2008, 8, 771–779. [DOI] [PubMed] [Google Scholar]
- 124.Danger G; Bossa J-B; de Marcellus P; Borget F; Duvernay F; Theulé P; Chiavassa T; d’Hendecourt L Experimental investigation of nitrile formation from VUV photochemistry of interstellar ices analogs: Acetonitrile and amino acetonitrile. Astron. Astrophys 2011b, 525, A30 (6pp). [Google Scholar]
- 125.Bell MB; Avery LW; MacLeod JM; Matthews HE The excitation temperature of HC9N in the circumstellar envelope. Astrophys. J 1992, 400, 551–555. [Google Scholar]
- 126.Öberg KI; Guzman VV; Furuya K; Qi C; Aikawa Y; Andrews SM; Loomis R; Wilner DJ The comet-like composition of a protoplanetary disk as revealed by complex cyanides. Nature 2015, 520, 198–201. [DOI] [PubMed] [Google Scholar]
- 127.Huang Y; Sachtler WMH Intermolecular hydrogen transfer in nitrile hydrogenation over transition metal catalysts. J. Catal 2000, 190, 69–74. [Google Scholar]
- 128.Engel MH; Macko SA Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [DOI] [PubMed] [Google Scholar]
- 129.Pizzarello S; Zolensky M; Turk KA Non racemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 2003, 67, 1589–1595. [Google Scholar]


