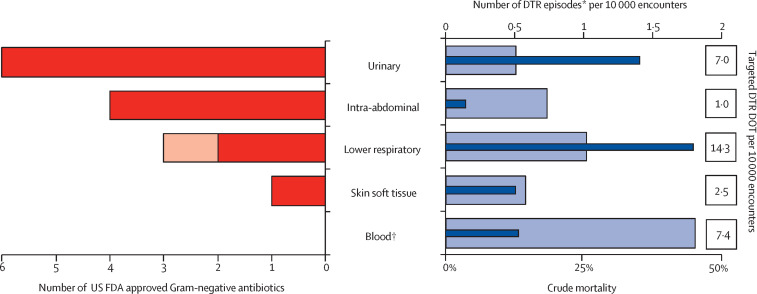
Figure 2.
DTR-GNI episodes, targeted treatment opportunities, and crude mortality versus number of US FDA approved Gram-negative active antibiotics by site since December, 2014
Thick bars on the left represent the number of FDA-approved Gram-negative active antibiotics by site. Both hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (dark red) and community-acquired pneumonia (light red) are displayed as lower respiratory. Thin dark blue bars on the right represent DTR episodes per 10 000 encounters by site and the light blue bars represent associated DTR mortality. FDA=Food and Drug Administration. DTR=difficult-to-treat resistance. DOT=days of therapy. *Includes all infection types per site; “other” infection site not included. †34 of 158 (21·5%) bloodstream infections are secondary to respiratory (n=17), urinary (n=14), skin and soft tissue (n=2), and lower respiratory (n=1) sites.