Figure 1.
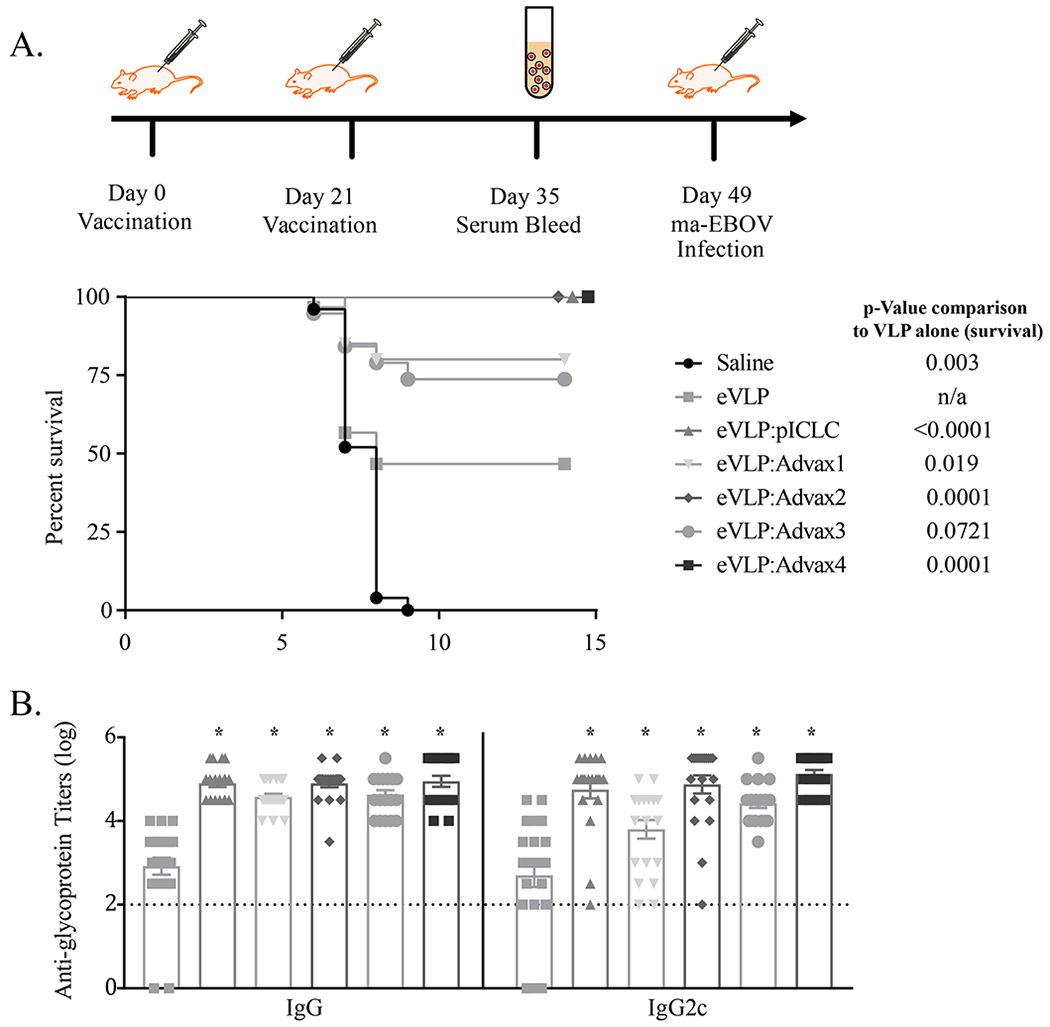
Vaccination with adjuvanted VLP GP antigen confers protection from Ebola virus challenge in mice. C57BL/6 mice were vaccinated with VLP (1.25 μg based on glycoprotein (GP) content) in combination with various adjuvants (1mg Advax-1, -2, -3, or -4 or 10 μg pICLC) on Day 0 and 21, and then challenged with 1000 pfu of mouse adapted (ma)-Ebola virus IP on day 49 (p-value based on Log-rank Mantel-Cox test) (A). Sera was collected from animals seven days after the second vaccination to assess anti-GP antibody titers; p value in comparison to eVLP alone indicated with asterisks (* = p<0.0001, one-way ANOVA comparison) (B). For saline, eVLP, eVLP + (pICLC, Advax-1, Advax-2, Advax-3, Advax-4) the number of animals was 20, 25, 20, 20, 20, 19, and 20, respectively, from combined studies. Animals vaccinated with adjuvant alone all succumbed to infection (data not shown).
