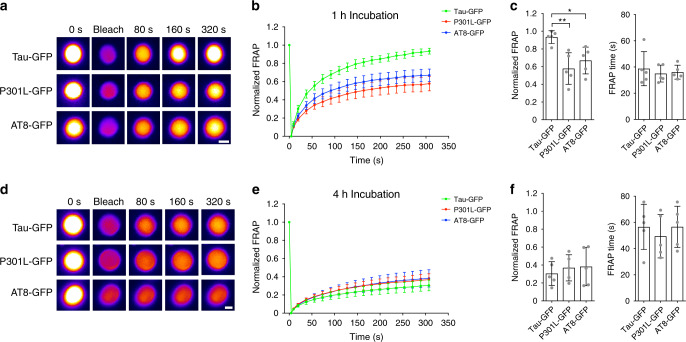
Fig. 5. Disease-related tau modifications form liquid droplet with reduced dynamic properties.
a Representative droplets of tau-GFP, P301L-GFP and AT8-GFP (4 µM each, 2 µM tau-GFP and 2 µM tau unlabeled) analyzed using FRAP after 1 h incubation in crowding conditions. b FRAP curves for each tau protein after 1 h incubation indicate that each protein shows dynamic exchange of proteins with the surrounding bulk phase (graph represents mean ± SEM). c Comparison of the final extent of recovery after photobleaching (i.e. 320 s) demonstrates that tau-GFP liquid droplets show significantly more recovery than P301L-GFP and AT8-GFP droplets after 1 h incubation (one-way ANOVA with Holm–Sidak post-hoc test, **p = 0.0054, *p = 0.0237, graph represents mean ± SD). The FRAP time was not statistically different between tau constructs (p = 0.24, one-way ANOVA). d Representative droplets of tau-GFP, P301L-GFP and AT8-GFP (4 µM each, 2 µM tau-GFP and 2 µM tau unlabeled) analyzed using FRAP after 4 h incubation in crowding conditions. e FRAP curves for each tau protein analyzed after 4 h incubation demonstrate marked reductions in the dynamic properties of droplets from all three tau proteins (graph represents mean ± SEM). f Comparison of the final extent of recovery after photobleaching (i.e. 320 s) for all tau liquid droplets show similar low levels of recovery after 4 h incubation (p = 0.30, one-way ANOVA, graph represents mean ± SD). The FRAP time was not statistically different between tau constructs (p = 0.31, one-way ANOVA). The 1 and 4 h FRAP experiments were repeated five independent times. Source data for b, c, e and f provided in the Source Data file.