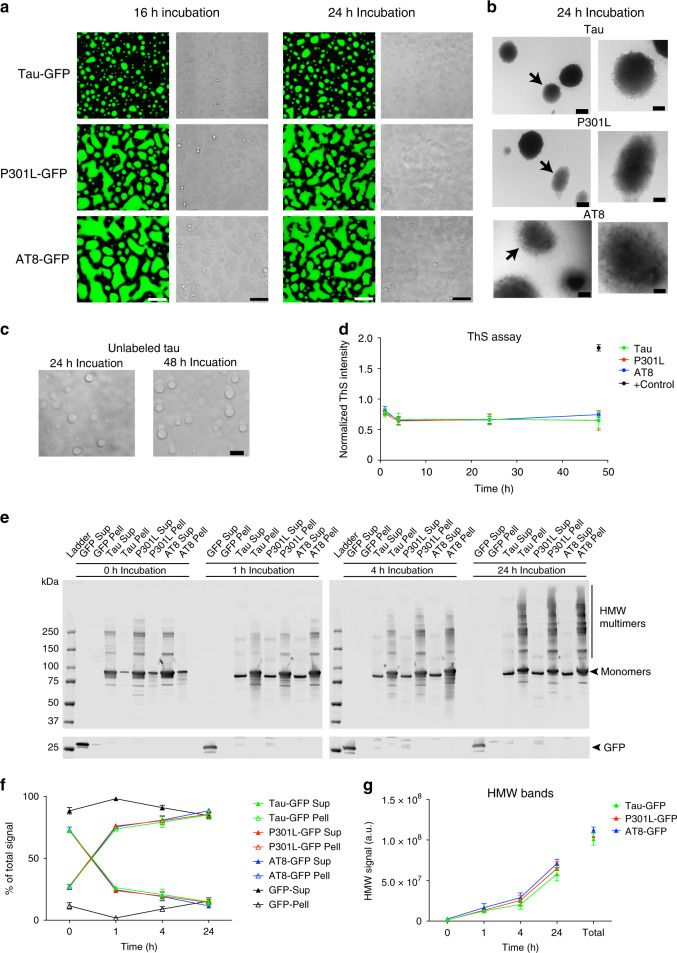
Fig. 6. Prolonged incubation of tau liquid droplets produces multimeric tau species but does not lead to fibril formation.
a Images of phase-separated structures formed by tau-GFP, P301L-GFP and AT8-GFP (each at 4 µM) after 16 and 24 h incubation at room temperature. Scale bar is 5 µm. b Transmission electron micrographs of phase-separated structures from unlabeled tau, P301L and AT8 proteins (4 µM each) after 24 h incubation at room temperature. Tau filaments were not readily apparent in the structures formed under these conditions (arrows indicate the droplet shown in the higher magnification images). Scale bars are 800 nm (left images) and 400 nm (right images). This experiment was repeated three independent times. c Incubation of unlabeled tau for 24 and 48 h produces droplets, not fibrillar structures, further confirming the lack of fibril formation via LLPS. d Thioflavin S (ThS), a fibrillar cross-β-sheet binding dye that labels tau filaments, showed low signal and no increase over time (1–48 h, data normalized to LLPS buffer with PEG blank) with prolonged phase separation confirming the lack of cross-β-sheet structures in tau proteins (e.g. filaments) (graph represents mean ± SD). Note that the positive control samples, arachidonic acid-induced tau aggregates (black circle), showed a robust increase in ThS intensity. e Supernatant (Sup) and pelleted (Pell) fractions from droplet spin down experiments were run in western blots to assess the time-dependent (0–24 h of phase separation) formation of heat-, reducing- and SDS-stable tau multimers. Notably, some high molecular weight tau species (HMW, i.e. tau multimers) were apparent shortly after addition of PEG (0 h time point). f Quantification of total tau in the Sup or Pell fractions shows a clear time-dependent shift in pelletable tau starting 1 h after phase separation is initiated and continuing through 24 h (graph represents mean ± SD). g Quantification of the HMW tau multimers shows a time-dependent increase in stable multimers with prolonged phase separation with all tau proteins (graph represents mean ± SD). Source data for d–g provided in the Source Data file.