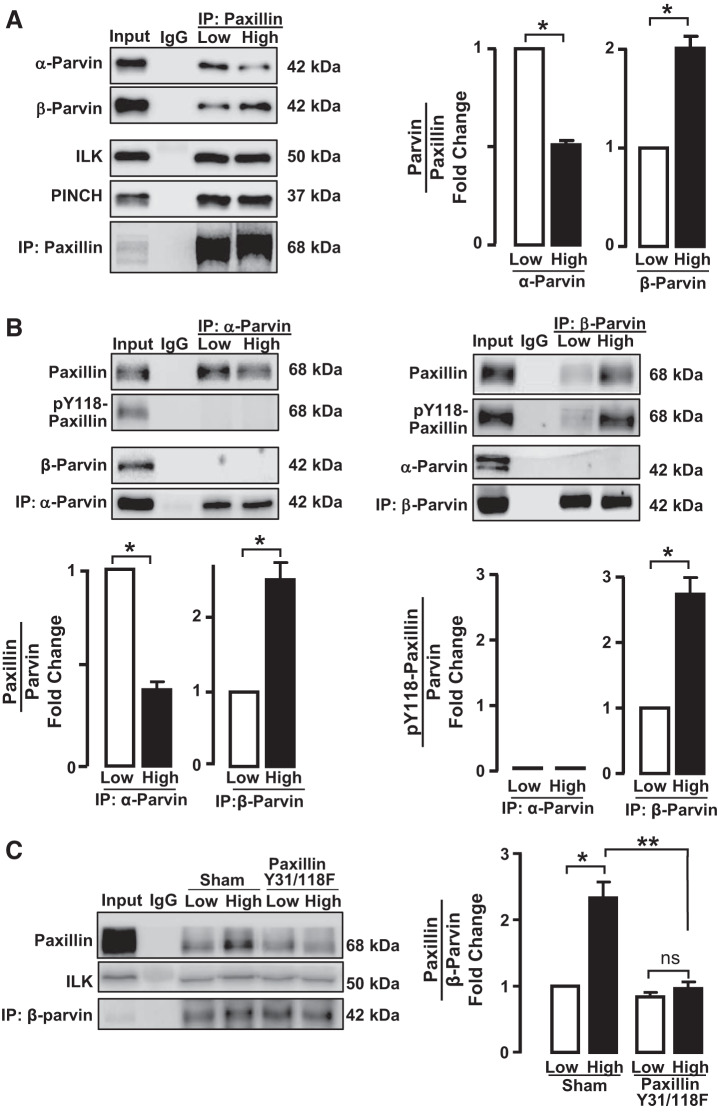
Fig. 3.
The interaction of α-parvin and β-parvin integrin-linked-kinase/PINCH/parvin (IPP) complexes with paxillin is differentially regulated by mechanical tension and is determined by paxillin phosphorylation. The effect of mechanical tension on the interaction of paxillin with α-parvin or β-parvin was determined by immunoprecipitation (IP) analysis of extracts of tracheal smooth muscle tissues incubated at low or high tension. A: paxillin immunocomplexes were analyzed for α-parvin, β-parvin, integrin-linked-kinase (ILK), and PINCH. Significantly more α-parvin precipitated with paxillin from tissues at low tension, and more β-parvin precipitated from tissues at high tension. No tension-dependent differences were found in the amount of ILK or PINCH in paxillin immunocomplexes (n = 4). B: α-parvin and β-parvin were immunoprecipitated from extracts of tissues maintained at low or high tension and immunocomplexes analyzed for the presence of α-parvin, β-parvin, paxillin, and phospho-paxillin. In α-parvin immunocomplexes, significantly more paxillin was detected in tissues at low tension (n = 4), whereas in β-parvin immunocomplexes significantly more paxillin was found at high tension (n = 8). Phospho-paxillin precipitated with β-parvin and was significantly higher in extracts from tissues at high tension (n = 3). Very little phospho-paxillin coprecipitated with α-parvin regardless of mechanical tension (n = 3). C: effects of inhibiting paxillin phosphorylation on the association of β-parvin with paxillin were analyzed in tissues in which paxillin phosphorylation was inhibited by expressing paxillin Y31/118F. The inhibition of paxillin phosphorylation suppressed the interaction between paxillin and β-parvin at high mechanical tension (n = 4). Data analyzed by one-way ANOVA. *Significant difference between low and high tension. **Significant difference between Sham-treated tissues and tissues expressing paxillin Y31/118F mutant. P < 0.05.