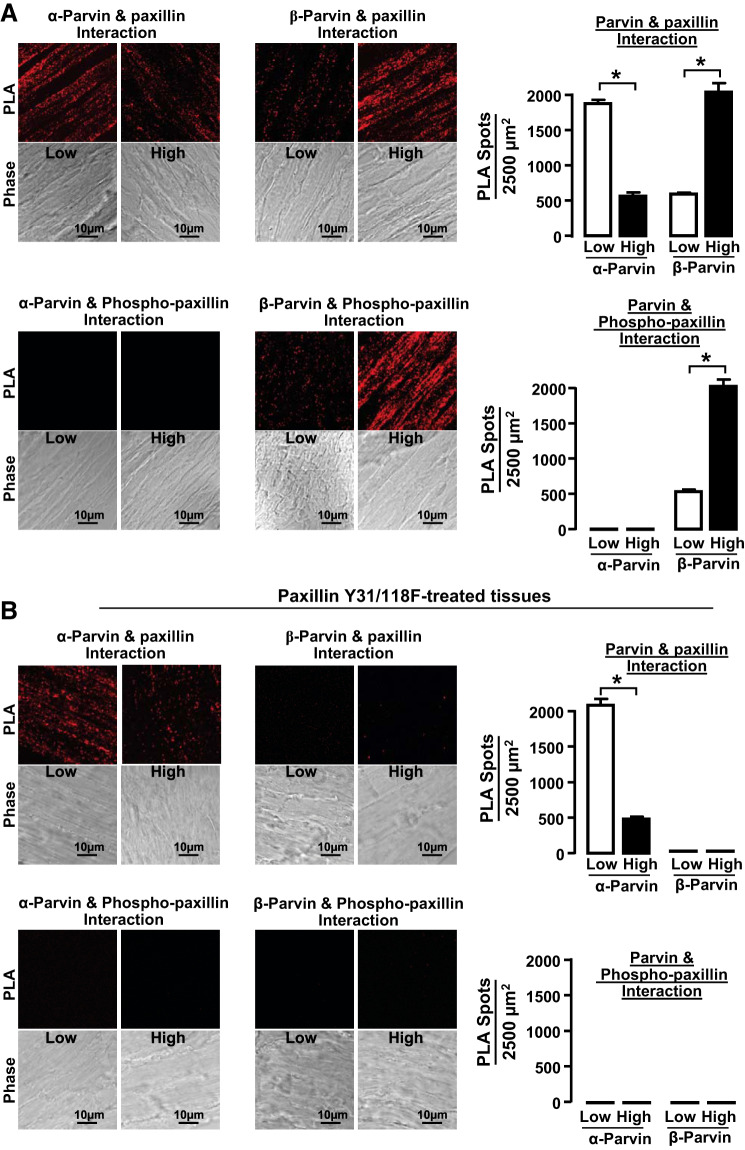
Fig. 4.
Low mechanical tension promotes interactions between α-parvin and unphosphorylated paxillin, whereas high mechanical tension promotes interactions between β-parvin and phosphorylated paxillin. A: cryo-sections from tissues maintained at low or high mechanical tension for 6 h showing proximity ligation assay (PLA) fluorescence from the interactions between α-parvin or β-parvin with paxillin or pY118-paxillin. More interactions between α-parvin and paxillin were detected in tissues at low mechanical tension, while more interactions between β-parvin and paxillin were detected in tissues at high mechanical tension. pY118-paxillin interacted with β-parvin and not with α-parvin regardless of mechanical tension (n = 9). B: cryo-sections from tissues maintained at low and high mechanical tension after transfection with paxillin Y31/118F to inhibit paxillin phosphorylation. PLA fluorescence for interactions between α-parvin and paxillin was similar to those in untreated tissues, whereas interactions between β-parvin and paxillin were inhibited in tissues treated with paxillin Y31/118F (n = 9). Data analyzed by one-way ANOVA. *Significant difference between low and high tension. P < 0.05.