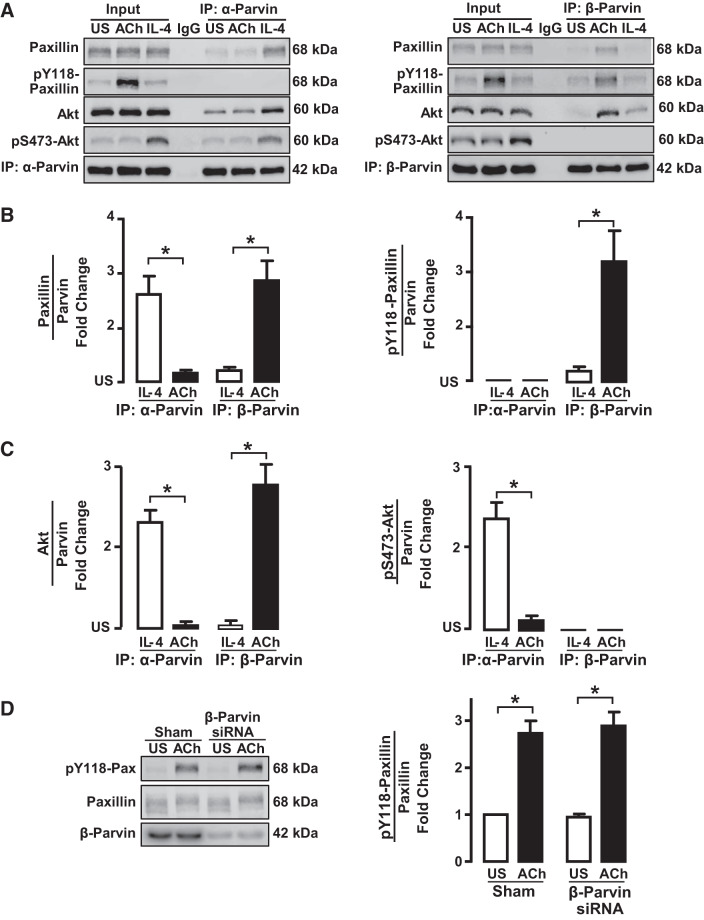
Fig. 8.
IL-4 stimulates the interaction of unphosphorylated paxillin with α-parvin, whereas ACh stimulates the interaction of phosphorylated paxillin with β-parvin. A: tissues were maintained isometrically and stimulated with ACh or IL-4 or were unstimulated (US). α-Parvin or β-parvin was immunoprecipitated (IP) from tissue extracts, and immunoprecipitates were blotted for paxillin and pY118-paxillin, Akt and pS473-Akt, and α-parvin or β-parvin. B: significantly more paxillin coprecipitated with α-parvin immunocomplexes from IL-4-stimulated muscles than from ACh-stimulated muscles or unstimulated US muscles (n = 6). Significantly more paxillin coprecipitated with β-parvin from ACh-stimulated muscles than from IL-4-stimulated or unstimulated muscles (n = 5). Paxillin that coprecipitated with α-parvin was not phosphorylated; phosphorylated paxillin only coprecipitated with β-parvin complexes (n = 5). C: significantly more Akt coprecipitated from α-parvin immunoprecipitates from tissues stimulated with IL-4 than from tissues stimulated with ACh or US tissues. In β-parvin immunoprecipitates, significantly more Akt was found in tissues stimulated with ACh than in tissues stimulated with IL-4 or US tissues. The Akt that precipitated with α-parvin was phosphorylated, but the Akt that coprecipitated with β-parvin was not phosphorylated (n = 5). D: tissues were depleted of β-parvin and stimulated with ACh. Similar amounts of phosphorylated paxillin were found in β-parvin-depleted tissues and Sham-treated tissues (n = 3). Statistical analysis by one-way ANOVA. *P < 0.05.