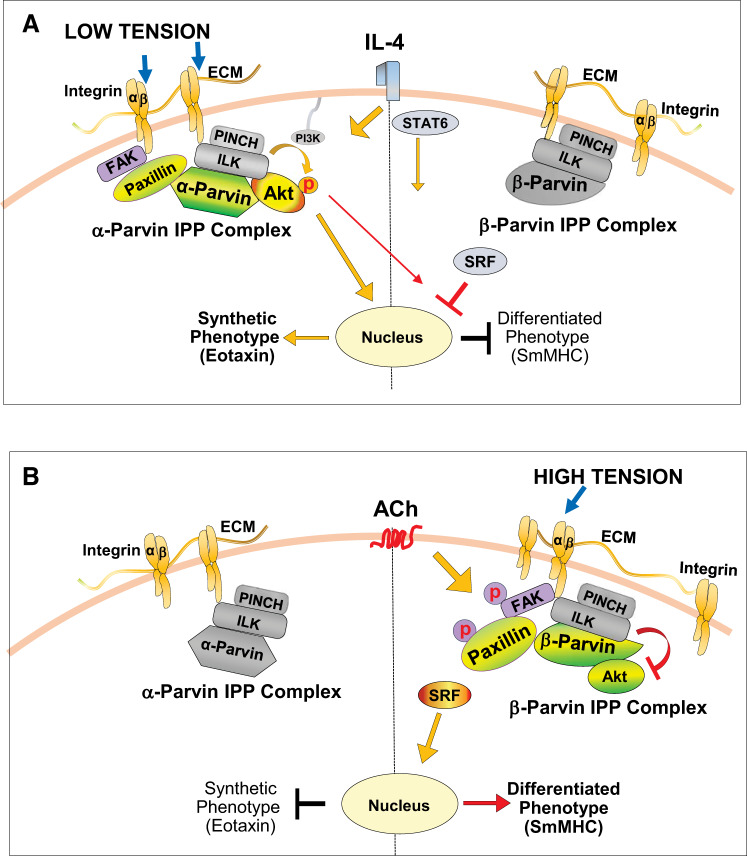
Fig. 12.
Proposed model for roles of α-parvin and β-parvin integrin-linked kinase/PINCH/parvin (IPP) complexes in phenotype modulation in airway smooth muscle. A: low mechanical tension or IL-4 stimulates the coupling of the α-parvin IPP complex to unphosphorylated paxillin within integrin adhesome complexes. This facilitates the recruitment of Akt to the α-parvin IPP complex, where it binds to integrin-linked kinase (ILK). The interaction of Akt with ILK promotes Akt activation by PI3-kinase and results in a synthetic inflammatory phenotype in the airway smooth muscle that is characterized by the synthesis of eotaxin. Akt activation suppresses the nuclear localization of serum response factor (SRF), which inhibits expression of the smooth muscle-specific marker protein, smooth muscle myosin heavy chain (SmMHC) (43). The β-parvin IPP complex also localizes to the membrane, but it does not bind to paxillin or couple to Akt. B: high mechanical tension or the stimulation of smooth muscle cells with ACh induces the β-parvin IPP complex to localize to membrane adhesomes, where it binds to tyrosine-phosphorylated paxillin. Akt preferentially binds to β-parvin rather than ILK within the β-parvin IPP complex. β-Parvin prevents the binding of Akt to ILK, which inhibits the activation of Akt. The inhibition of Akt activation promotes the nuclear localization of SRF and expression of the differentiated smooth muscle phenotype. ECM, extracellular matrix; FAK, focal adhesion kinase.