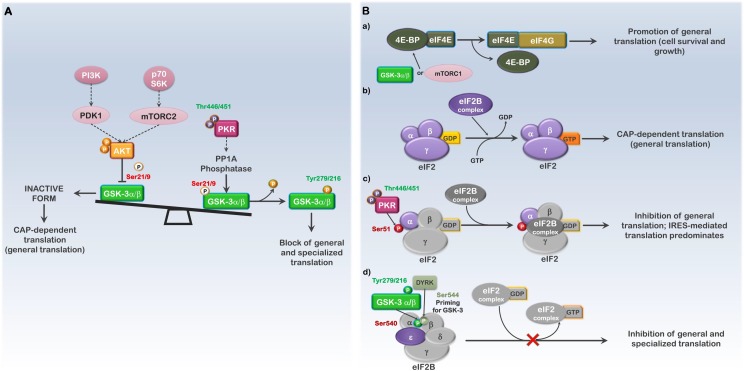
Fig. 1.
GSK3α/β and PKR regulate translation initiation. The diagram shows the effects of GSK3α/β and PKR activity on translation initiation and the various potential outcomes of kinase-dependent modifications on translation. (A) Regulation of the PI3K-AKT-mTOR and PKR pathways can influence translation initiation through modulation of GSK3 activity. (B) Activation of the PI3K-AKT (mTOR or GSK3α/β) and PKR pathways affect the eIF4 and eIF2 translation initiation complexes. a; GSK3α/β- or mTOR- mediated phosphorylation of 4E-BP inhibits its association with eIF4E, promoting general translation. b; The eIF2B GDP-GTP exchange factor complex (eIF2B complex) must replace GDP bound to the eIF2 translation initiation complex (eIF2) with GTP in order to initiate each new round of CAP-dependent (general) translation. c; Active PKR (phospho-T446/T451) results in eIF2α phosphorylation on S51 and the inhibition of general translation by stabilizing the eIF2:eIF2B-GDP interaction; IRES-mediated translation of stress-response genes is favored. d; Active GSK3α/β (phospho-Y279/216) phosphorylates the ε subunit of the eIF2B complex on S540, resulting in inhibition of both general and specialized (IRES-mediated) translation. While active PKR is denoted as phosphorylated on T446 and T451, only T451 is required for activity. Phosphorylation of T446 as well as numerous additional sites influences the degree of activation. Phosphorylation sites labeled in green (ex., Tyr216-GSK3β) indicate activating or stabilizing modifications; phosphorylation sites labeled in red (ex., Ser51-eIF2α) indicate modifications that are inhibitory to the substrates activity.