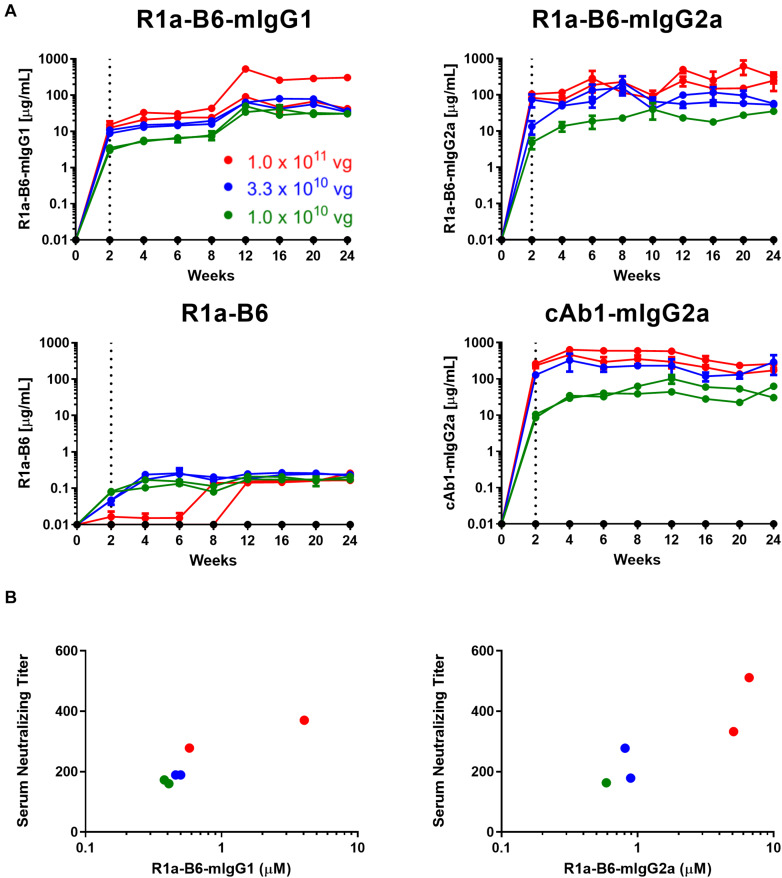
FIGURE 2.
AAV dose ranging study of anti-influenza neutralizing nanobody R1a-B6 in mice. (A) Different R1a-B6 constructs and the control nanobody, cAb1, were given via AAV in vector genome (vg) doses of 1.0 × 1010 vg (green series), 3.3 × 1010 vg (blue series), and 1.0 × 1011 vg (red series). Mice that were given PBS are indicated by the black series. Nanobodies in serum of BALB/c mice were measured from week 0 to 24. A mouse given 1.0 × 1010 vg of R1a-B6-mIgG2a was culled before the end of the study due to reasons exclusive of AAV delivery. Each individual series corresponds to a single mouse. The dotted line represents the point (2 weeks) at which nanobody expression levels were first detected. (B) Neutralizing activity of sera taken from mice 24 weeks after they were injected with different doses of AAV encoding R1a-B6-mIgG1 and R1a-B6-mIgG2a was measured against 103 TCID50/mL of CA/09. The serum neutralizing titer is expressed as the reciprocal of the highest dilution at which influenza infection is completely blocked. Mice given R1a-B6 (all doses) did not show any neutralizing activity (data not shown). For all plots, each point represents an individual mouse serum sample, n = 2 mice/group.