Abstract
Purpose
To describe chronic performance of subxiphoid minimally invasive pacemaker lead insertion in a piglet model.
Methods
Minimally invasive pacemaker lead implantation was performed through a 10-mm incision under direct visualization using the PeriPath port. Epicardial access was obtained and the commercially available Medtronic Model 20066 pacemaker lead was inserted into the pericardial space and epicardial fixation was performed using the side-action helix. The lead was connected to a pacemaker generator in a para-rectus pocket. Animals underwent a 12–14-week observation period and lead impedances, R-wave amplitudes, and ventricular capture thresholds were tested biweekly. After the survival period, animals were euthanized and gross and histopathology were performed.
Results
Subxiphoid minimally invasive pacemaker lead placement was performed in 8 animals (median 4.9 kg) with 100% acute success. Median procedure time was 65 min (IQR 60.5–77). At implant, median lead impedance was 650 Ω (IQR 244–984), R-wave amplitude 11.1 mV (IQR 8–12.3), and ventricular capture threshold 1.5 V @ 0.4 ms (IQR 1–2.6). Over a median survival period of 13 weeks, there was a median lead impedance change of + 262 Ω (IQR 5.3–618.3), R-wave change of − 4.5 mV (IQR −7.1–− 2.7) and capture threshold change (1.0 ms) of + 1.5 V (IQR 0–3.3). At autopsy, epicardial fixation sites showed fibrovascular proliferation and minimal chronic inflammation.
Conclusions
Subxiphoid pericardial pacemaker placement is safe and effective in a piglet model. Further study and development of leads designed for pericardial placement are warranted.
Keywords: Pacemaker, Pericardial, Subxiphoid, Minimally invasive, Animal model
1. Introduction
Advanced heart block, including high-grade 2nd-degree or 3rd-degree block, in pediatric patients is relatively rare but often requires the placement of a permanent pacemaker (PM). The most common causes of advanced heart block in pediatric and adult congenital heart disease (CHD) patients are postoperative from repair of CHD and auto-immune secondary to maternal systemic lupus erythematous (SLE) and Sjogren’s syndrome. Heart block due to SLE and Sjogren’s syndrome occurs as the result of the maternal transfer of anti-Ro/SSA antibodies that affect the conduction system in-utero and has a reported incidence ranging from 1 to 2% [1]. Heart block in infancy often requires placement of a permanent PM. In a cohort of 19 patients with auto-immune heart block, 89% required PM placement with 82% of those patients requiring PM placement within the first year of life [2]. The incidence of postoperative high-grade heart block ranges from 2.7 to 4.1% and has been shown to be associated with increased risk of mortality [3, 4]. Requirement of PM placement for postoperative heart block has been reported at an incidence of 1% of surgically repaired CHD [3, 4].
Infants, small children, and patients with certain types of repaired CHD are not candidates for transvenous PM placement secondary to inadequate vessel size, venous obstruction, endocarditis, atrial or ventricular septal defects, or inadequate intra-cardiac access [5]. Epicardial lead placement often requires a larger incision, either subxiphoid, thoracotomy, or sternotomy, and can be associated with increased pain, cost, morbidity, and longer length of stay. Further, epicardial placement creates further difficulty given the presence of adhesions that increase the risk of subsequent procedures [6].
Multiple authors have described the use of the pericardial space for device lead placement in addition to other uses [7]. Prior work by our team demonstrated the feasibility of left ventricular (LV) PM and pericardial ICD lead placement in an infant animal model utilizing a minimally invasive approach [8, 9]. The initial approach involved subxiphoid needle pericardial access with lateral thoracoscopic visualization. Development of PeriPath [10], a novel device that incorporates access and visualization, allowed for subxiphoid pericardial ICD lead placement in an infant piglet model [11]. Our initial experience with subxiphoid ICD lead placement demonstrated the feasibility of placement and chronic lead performance during a short survival period. The number of procedures performed was small and survival period was limited. This prior work included an ICD lead specifically designed for pericardial placement and epicardial fixation, one that is not commercially available. The goal of this study was to expand on our prior work by evaluating the chronic performance of the pericardial placement of a derivative of a commercially available (non-prototype) PM lead in a larger number of animals over a longer period of follow-up.
2. Methods
All animal implant procedures and follow-up care were performed at SoBran BioScience (Browns Summit, NC). The project was approved by the local Institutional Animal Care and Use Committee at SoBran BioScience and the study was performed under good laboratory practice (GLP) conditions. All procedures were performed on the Sinclair species of piglet given a slower weight gain curve compared to other species. A piglet model was utilized based on the similarity of cardiac anatomy and coronary vasculature that has been described previously [12]. Animals were brought to the operative room in the post-absorptive state. Intravenous access was obtained, the piglets were intubated and general anesthesia was maintained throughout the procedure. The subxiphoid and para-rectus areas were prepped and sterilized with betadine solution. A small subcutaneous right-sided para-rectus pocket, anterior to the musculature, was created using a combination of blunt dissection and electrocautery.
After pocket completion, a 10-mm incision was made in the subxiphoid area. Using blunt dissection, the incision was carried to the level of the diaphragm and the diaphragm was pierced using a blunt-tipped instrument. Figure 1 demonstrates the procedural workflow from the exterior of the animal. The PeriPath port (PeriCor LLC, Bethesda, MD, USA) was inserted into the incision, a rigid thoracoscope (EndoCAMeleon, KARL STORZ, El Segundo, CA) inserted through the larger channel of the PeriPath device and the left thoracic cavity was insufflated with CO2. After visualization of the heart, a pericardiocentesis needle was inserted into the smaller port of the PeriPath and the needle tip visualized. The needle was utilized to pierce the pericardium and a small amount of saline was infused through the needle to hydrodissect the pericardial space. A pericardiocentesis wire was inserted through the needle until it was visualized and confirmed within the pericardial space. The needle was removed and a tear-away sheath (7F-25 mm, Merit Medical, South Jordan, UT, USA) was inserted over the wire and the distal sheath placement within the pericardial space was confirmed. The wire and dilator were then removed.
Fig. 1. a.
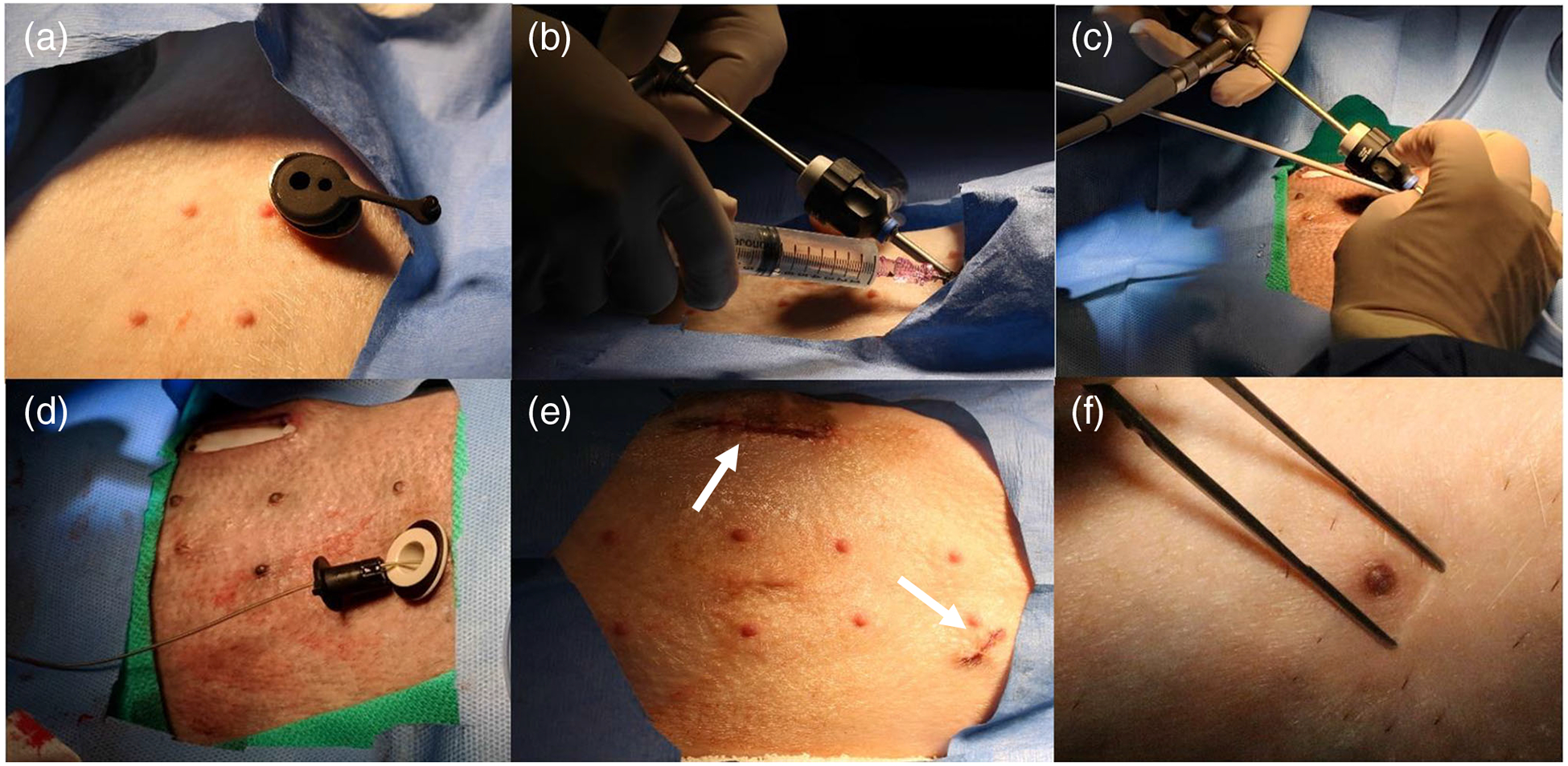
PeriPath in place within subxiphoid incision, b thoracoscope and needle in place within PeriPath, c sheath and dilator insertion, d lead placement after sheath and thoracoscope removal, e subxiphoid and para-rectus incisions after procedure completion, f healed subxiphoid incision after completion of survival period
Under direct visualization, the PM lead (Fig. 2) was inserted through the sheath and into the pericardial space. The Model 20066 left ventricular lead (Medtronic Inc., Minneapolis, MN) is a 4-F bipolar, steroid eluting, active fixation lead that is a derivative of the market released Attain Ability Model 4196 lead (Medtronic Inc., Minneapolis, MN) [13]. The lead has a small exposed side-helix for fixation with mechanical stop that avoids over-torquing [14]. Thoracoscopic visualization was utilized in all cases to guide fixation to-wards the left ventricle and to confirm that there were no coronary vessels present at the lead fixation site. After confirmation of proper location, the lead was fixated to the epicardial surface of the heart using side-action fixation from manual torque of the proximal portion of the lead. Gentle backwards pressure was placed on the proximal portion of the lead to confirm fixation without lead movement. After fixation, lead measurements were performed including impedance, R-wave amplitude, and ventricular capture threshold. Once adequate ventricular sensing and capture thresholds were confirmed, the tear-away sheath was removed leaving only the PM lead remaining in the pericardial space. Thoracoscopic visualization confirmed lead placement and no evidence of pericardial effusion. Figure 3 demonstrates the major steps of the procedure seen by direct thoracoscopic visualization.
Fig. 2.

Model 20066 left ventricular pacemaker lead with zoomed up image of side-action helix for epicardial fixation. *Reproduced with permission of Medtronic, Inc.
Fig. 3.
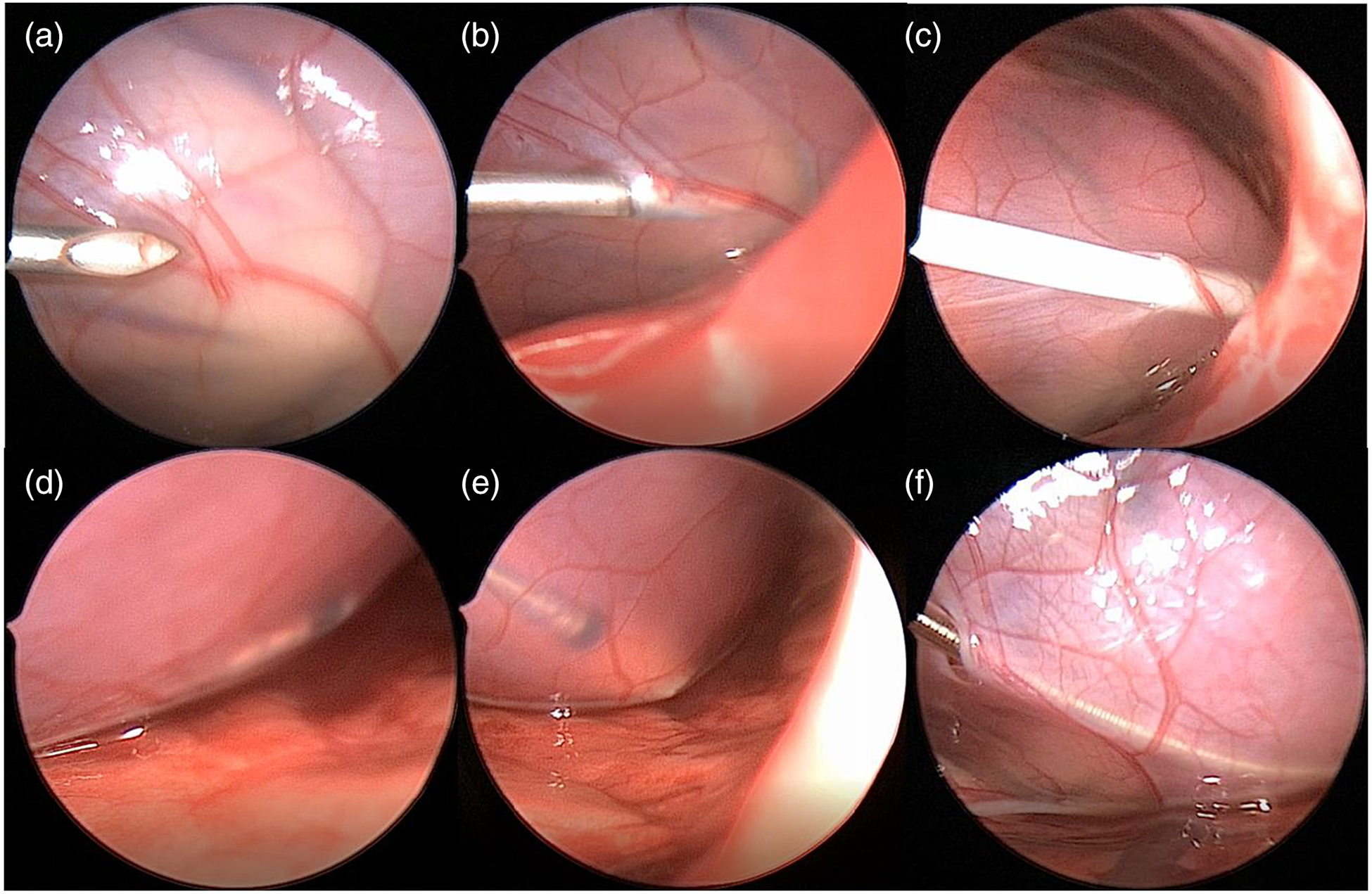
Procedural workflow as seen by thoracoscopic visualization a needle visualization, b needle insertion into pericardial space, c successful sheath insertion into pericardial space, d distal pacemaker lead placement within pericardial space, e zoomed up image of pacemaker lead tip, f completed procedure with lead in place and small defect remaining in pericardium
After sheath removal, the PM lead was tunneled subcutaneously to the right-sided para-rectus pocket and connected to the PM generator (Adapta, Medtronic Inc., Minneapolis, MN, USA). Device interrogation was performed with the generator in the para-rectus pocket and adequate impedance, sensing, and capture threshold values were confirmed. Multi-layer suture closure was then performed of both the para-rectus abdominal pocket and subxiphoid incision. Piglets were then extubated and re-covered from anesthesia. All animals underwent a survival period that ranged from 12 to 14 weeks. During the survival period, devices were interrogated every other week for lead impedance, R-wave amplitude, and capture threshold. Incision sites and animals were checked daily to evaluate for signs of infection or distress. At the end of the survival period, repeat device interrogation was performed to evaluate lead impedance, ventricular sensing and capture thresholds. After repeat testing was completed, humane euthanasia was performed. Gross and histopathology were performed under GLP conditions by an independent reviewer (Histo-Scientific Research Laboratories, Mount Jackson, VA) at the following areas: subxiphoid incision, diaphragmatic insertion site, lead tract, and epicardial fixation site. Quantification of fibrovascular proliferation, fibrosis, and inflammation was performed on each specimen by the histopathologist and was made subjectively on a scale ranging from minimal to severe.
3. Results
Minimally invasive Model 20066 pericardial PM lead placement was performed in 8 animals with 100% acute success. There were no complications related to epicardial access or pacemaker lead implantation. Median weight at placement was 4.9 kg (IQR 3.9–6.6). Procedure timing values are presented in Table 1. The incision length ranged from 10 to 13 mm. Lead impedance, R-wave amplitude values, and capture thresholds (both at 0.4 ms and 1.0 ms) at implant and at the end of the survival period are presented in Table 2. At initial implant, one animal could not capture at a pulse width of 0.4 ms and required 5 V @ 1.0 ms for capture. Median length of the survival period was 13 weeks (IQR 12–14) and 50% of animals completed the entire 14-week survival period. One animal required early euthanasia at 8 weeks secondary to pocket infection; ventricular capture threshold at the time of euthanasia was 1.0 V @ 0.4 ms. There were no other associated morbidities in the remaining animals. Median weight at the end of the survival period was 15.8 kg (IQR 11.9–17.6) and the median percentage weight gain during that period was 287% (IQR 251–374). Of the animals, 5/8 could not achieve ventricular capture at 0.4 ms at the end of the survival period and only 1 animal could not achieve capture at 1.0 ms. Of the animals that could not capture at 0.4 ms at 14 weeks, 3 animals were able to capture at a range of 4.5–5.0 V @ 0.4 ms at 12 weeks.
Table 1.
Procedural timing for Model 20066 lead implantations
| Procedure | Timing (minutes) (median, IQR) |
|---|---|
| Total procedure time | 65, 60.5–77 |
| Pocket creation | 8.5, 5.8–12.3 |
| Time to needle visualization | 9, 4.8–13.3 |
| Time to pericardial access | 16, 14.3–19.5 |
| Time to lead implantation | 24, 22–30.8 |
Table 2.
Implant and end-of-survival period lead measurements
| Implant (median, IQR) | End-of-survival period (median, IQR) | Change (median, IQR) | |
|---|---|---|---|
| Lead impedance (ohms) | 650 (244–984) | 1188 (659–1268) | 262 (5.3–618.3) |
| R-wave amplitude (mV) | 11.1 (8.0–12.3) | 4.8 (3.7–8.8) | − 4.5 (− 7.1–−2.7) |
| Capture threshold (V @0.4 ms) | 1.5 (1.0–2.6) | 3.5 (2.3–3.6) | 0.5 (0.3–1.6) |
| Capture threshold (V @1.0 ms) | 1.3 (0.5–1.5) | 4.5 (3.1–5.0) | 1.5 (0–3.3) |
Necropsy was performed and epicardial lead fixation was confirmed with evidence of capsular formation around the lead between the diaphragmatic entry site and pericardium (Fig. 4). At autopsy, all subxiphoid incisions were healed with evidence of re-epithelialization and underlying fibrosis. Defects in the diaphragm were noted to have fibrosis, minimal to mild myofiber atrophy, minimal chronic inflammation, and presence of fibrovascular tissue, but no residual defect was noted. The lead tract at the diaphragmatic entry sites was noted to have granulomatous inflammation. Epicardial lead fixation sites were marked by mild fibrovascular proliferation, mild to moderate fibrosis on the epicardial or pericardial surface, and minimal to mild chronic inflammation. Adhesions between the epicardial and pericardial surfaces were noted in 75% of the animals that were evaluated.
Fig. 4.
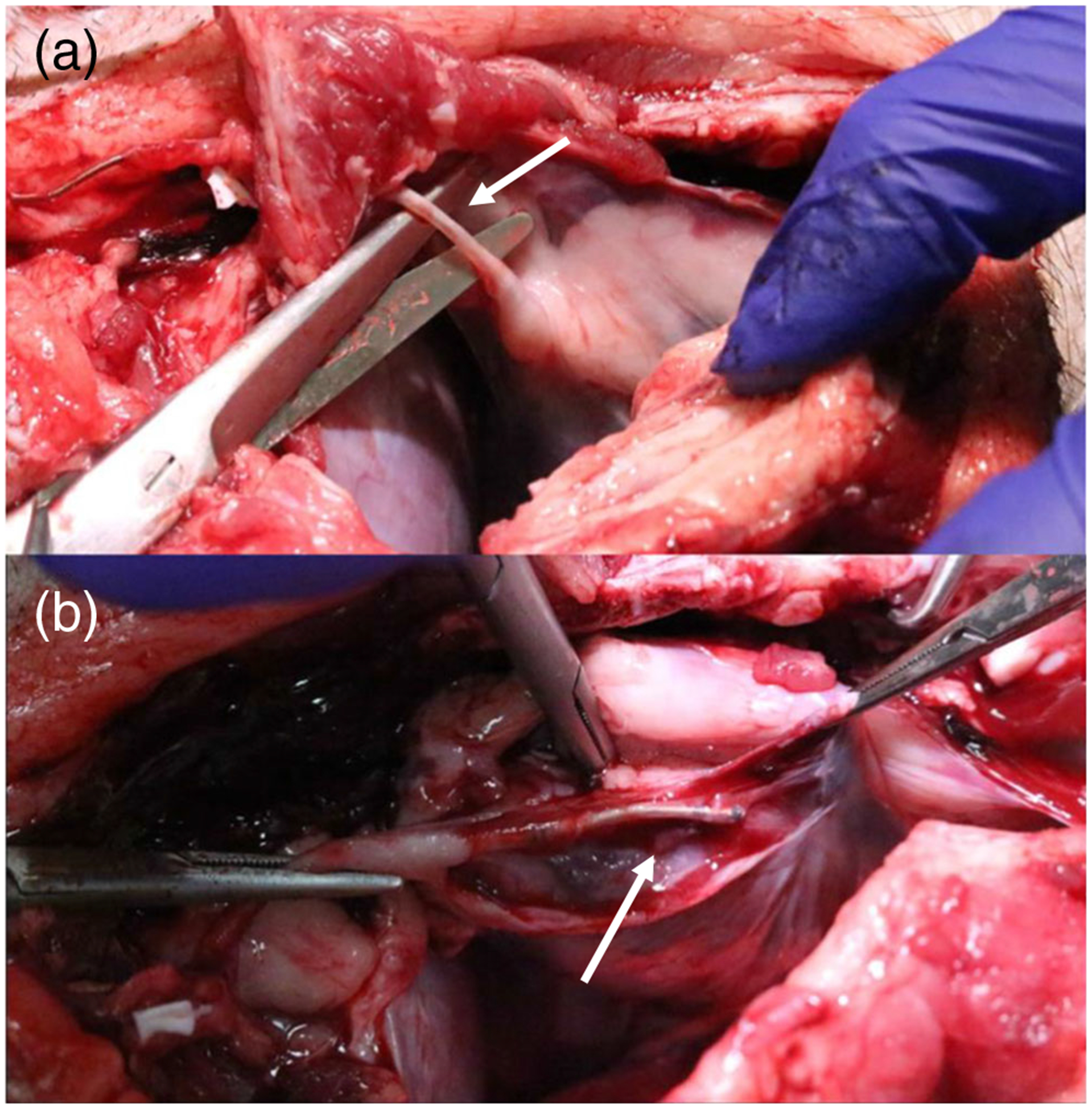
Gross appearance of a lead tract from diaphragmatic defect to pericardial space and b area of epicardial fixation and lead within the pericardial space
4. Discussion
We present the first report of minimally invasive pericardial insertion of a derivative of a commercially available (outside of the USA) PM lead in an animal model. Our group has previously demonstrated the feasibility of minimally invasive pericardial ICD lead placement, though the prototype lead was specifically engineered and designed for pericardial placement and epicardial fixation. The description of subxiphoid minimally invasive ICD lead insertion demonstrated that the procedure was safe and efficacious but the number of animals performed was small and there were limited survival periods [15]. All lead placements in this series were acutely successful utilizing a single small subxiphoid incision (maximum 13 mm). The median time of the procedure was 65 min, including pocket creation and skin closure of multiple incisions, and each procedure was performed by a pediatric electrophysiologist or pediatric cardiology fellow. There were no acute complications related to the procedure and most animals completed the full survival period with a single animal requiring early euthanasia at 8 weeks secondary to infection.
While lead impedance and ventricular sensing thresholds were relatively stable throughout the survival period, there was substantial increase in capture threshold including some animals that could not achieve capture at the end of the survival period. Multiple factors could contribute to the loss of capture at the end of the survival period. We utilized a derivative of the Attain Stability PM lead which is designed for transvenous use in the coronary sinus and not specifically for epicardial fixation. Additionally, the animals averaged a nearly threefold increase in their weight over a 3-month period which is not representative of a typical human patient. There is a concern that inflammation and fibrosis at the fixation site could have led to these capture threshold increases based on histopathologic demonstration of mild to moderate fibrosis, though only minimal to mild chronic inflammation at the fixation site was noted.
Multiple authors have described variants of minimally invasive or pericardial lead placement in both human and animal models. Hatam et al. described obtaining pericardial access using endoscopy and a 10-mm subxiphoid incision and they were successful in implanting PM leads in 80-kg adult pigs [16]. Others have described either video-assisted thoracoscopic surgery (VATS) or video-assisted PM lead placement through thoracoscopic ports [17, 18]. Costa et al. describe using either a 3-cm subxiphoid incision for neonatal PM lead placement and 5-cm subxiphoid incision and pericardial window creation and fluoroscopic guidance for adult PM lead placement [19, 20]. The pericardial space is becoming more frequently utilized with novel devices being created for specific placement in the pericardial space. Bar-Cohen and colleagues have described a novel fetal micropacemaker with the entire device placed within the pericardial space [21]. Kumthekar et al. has recently described utilizing a PM leadlet placed in the pericardial space connected to a Micra™ (Medtronic Inc., Minneapolis, MN) generator placed in the subxiphoid space, all performed through a 1-cm subxiphoid incision [22].
The minimally invasive subxiphoid approach to PM lead placement is feasible, safe, and reproducible. We have described the successful acute placement by multiple different operators, including a trainee and junior pediatric electrophysiologist. The authors believe that the subxiphoid approach, use of the novel PeriPath port, and epicardial access for lead implantation are equivalent to current approaches but there are clearly concerns about long-term lead stability using an epicardial fixation approach. Unfortunately, due to the nature of the necropsy procedure, the leads were not made available and evaluated for structural damage to explain the changes in sensing and capture thresholds. While the authors maintain that the leads were stable throughout the survival period based on impedance and lead location on gross pathology, radiographic evaluation was not available, so a lack of lead migration could not be proven. There was an animal that developed an infection and required early euthanasia, so the adherence to sterile techniques remains paramount. Further study, especially in patients with prior history of surgery for CHD and concern for presence of adhesions is certainly warranted. The design of a PM or ICD lead that is specifically designed for pericardial placement and epicardial fixation will hopefully improve performance and durability with this approach. While the Model 40066 PM lead is not available in the USA, there is a quadripolar version that is currently undergoing a clinical trial.
5. Conclusions
We have demonstrated the safety and feasibility of subxiphoid minimally invasive pericardial placement and chronic performance of a derivative of a commercially available PM lead in an infant animal model. Lead placement was acutely successful and the entire procedure was completed in approximately 1 h in most animals. The lead demonstrated stable lead impedance and ventricular sensing thresholds though there was a progressive increase in capture thresholds during the survival and growth period. Further study and design of a PM lead designed for the specific purpose of pericardial placement and epicardial fixation are warranted.
Acknowledgments
The authors would like to thank Mark Marshall with Medtronic Inc. for his continued assistance with pacemaker leads and technical support, David S. Garlick, DVM for pathologic evaluations, and Ashley Dean, Madison Saunders, Brittany Hardiman, Charlie Cook, and Gary Wolfe with SoBran BioScience for their assistance with animal procedures and follow-up care.
Funding information Funding for this project was provided by the Van Metre Companies Endowed Chair and philanthropic donations.
Footnotes
Conflict of interest Bradley Clark, Justin Opfermann and Charles Berul have intellectual property interests in PeriPath and are co-founders of PeriCor, LLC. Rohan Kumthekar and Paige Mass have no conflicts of interest to disclose related to this manuscript.
Statement on welfare of animals All applicable international, national, and institutional guidelines for the care and use of animals were followed. The project was approved by the local Institutional Animal Care and Use Committee and the study was performed under good laboratory practice (GLP) conditions.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brucato A, Cimaz R, Caporali R, Ramoni V, Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. 2011;40(1):27–41. 10.1007/s12016-009-8190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DE Caluwé E, Van De Bruaene A, Willems R, Troost E, Gewillig M, Rega F, et al. Long-term follow-up of children with heart block born from mothers with systemic lupus erythematosus: a retrospective study from the database pediatric and congenital heart disease in University Hospitals Leuven. Pacing Clin Electrophysiol. 2016;39(9):935–43. 10.1111/pace.12909. [DOI] [PubMed] [Google Scholar]
- 3.Romer AJ, Tabbutt S, Etheridge SP, Fischbach P, Ghanayem NS, Reddy VM, et al. Atrioventricular block after congenital heart surgery: analysis from the Pediatric Cardiac Critical Care Consortium. J Thorac Cardiovasc Surg. 2019;157(3):1168–77.e2. 10.1016/j.jtcvs.2018.09.142. [DOI] [PubMed] [Google Scholar]
- 4.Liberman L, Silver ES, Chai PJ, Anderson BR. Incidence and characteristics of heart block after heart surgery in pediatric patients: a multicenter study. J Thorac Cardiovasc Surg. 2016;152(1):197–202. 10.1016/j.jtcvs.2016.03.081. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt AB, Foster E, Kuehl K, Alpert J, Brabeck S, Crumb S et al. Congenital heart disease in the older adult: a scientific statement from the American Heart Association. 2015. [DOI] [PubMed]
- 6.Nkere UU. Postoperative adhesion formation and the use of adhesion preventing techniques in cardiac and general surgery. ASAIO J. 2000;46(6):654–6. [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Miyoshi S, Okamoto K, Fukumoto K, Tanimoto K, Soejima K, et al. The effectiveness of rigid pericardial endoscopy for minimally invasive minor surgeries: cell transplantation, epicardial pacemaker lead implantation, and epicardial ablation. J Cardiothorac Surg. 2012;7(1):117 10.1186/1749-8090-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan CP, Wu K, Costello JP, Ishibashi N, Krieger A, Kane TD, et al. Minimally invasive resynchronization pacemaker: a pediatric animal model. Ann Thorac Surg. 2013;96(6):2210–3. 10.1016/j.athoracsur.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 9.Clark BC, Davis TD, El-Sayed Ahmed MM, McCarter R, Ishibashi N, Jordan CP, et al. Minimally invasive percutaneous pericardial ICD placement in an infant piglet model: head-to-head comparison with an open surgical thoracotomy approach. Heart Rhythm. 2015;13(5):1096–104. 10.1016/j.hrthm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Opfermann JD, Clark BC, Davis TD, Berul CI, Krieger A. A single-incision delivery tool for epicardial pacing and defibrillation. J Med Devices Trans ASME. 2016;10:2 10.1115/1.4033123. [DOI] [Google Scholar]
- 11.Clark BC, Opfermann JD, Davis TD, Krieger A, Berul CI. Single-incision percutaneous pericardial ICD lead placement in a piglet model. J Cardiovasc Electrophysiol. 2017;28:1098–104. 10.1111/jce.13263. [DOI] [PubMed] [Google Scholar]
- 12.Crick SJ. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193:105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yee R, Gadler F, Hussin A, Bin Omar R, Khaykin Y, Verma A, et al. Novel active fixation mechanism permits precise placement of a left ventricular lead: early results from a multicenter clinical study. Heart Rhythm. 2014;11(7):1150–5. 10.1016/j.hrthm.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Eijkemans MJC, van Houdenhoven M, Nguyen T, Boersma E, Steyerberg EW, Kazemier G. Predicting the unpredictable. Anesthesiology. 2010;112(1):41–9. 10.1097/ALN.0b013e3181c294c2. [DOI] [PubMed] [Google Scholar]
- 15.Clark BC, Krieger A, Ms JDO, Berul CI, Davis TD. Single-incision percutaneous pericardial ICD lead placement in a piglet model. 2017:1–7. 10.1111/jce.13263. [DOI] [PubMed]
- 16.Hatam N, Amerini AL, Steiner F, Lazeroms M, Mischke K, Schauerte P, et al. Video-assisted pericardioscopic surgery: refinement of a new technique for implanting epimyocardial pacemaker leads. Eur J Cardiothorac Surg. 2011;39(3):335–41. 10.1016/j.ejcts.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Amraoui S, Labrousse L, Sohal M, Jansens JL, Berte B, Derval N, et al. Alternative to left ventricular lead implantation through the coronary sinus: 1-year experience with a minimally invasive and robotically guided approach. Europace. 2017;19(1):88–95. 10.1093/europace/euv430. [DOI] [PubMed] [Google Scholar]
- 18.Jaroszewski DE, Altemose GT, Scott LR, Srivasthan K, DeValeria PA, Lackey J, et al. Nontraditional surgical approaches for implantation of pacemaker and cardioverter defibrillator systems in patients with limited venous access. Ann Thorac Surg. 2009;88(1): 112–6. 10.1016/j.athoracsur.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Costa R, Silva KRD, Martinelli Filho M, Carrillo R. Minimally invasive epicardial pacemaker implantation in neonates with congenital heart block. Arq Bras Cardiol. 2017;109(4):331–9. 10.5935/abc.20170126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa R, Scanavacca M, da Silva KR, Martinelli Filho M, Carrillo R. Novel approach to epicardial pacemaker implantation in patients with limited venous access. Heart Rhythm. 2013;10(11):1646–52. 10.1016/j.hrthm.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Bar-Cohen Y, Silka MJ, Hill AC, Pruetz JD, Chmait RH, Zhou L, et al. Minimally invasive implantation of a micropacemaker into the pericardial space. Circ Arrhythm Electrophysiol. 2018;11(7): e006307 10.1161/circep.118.006307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumthekar RN, Opfermann JD, Mass P, Clark BC, Moak JP, Sherwin ED, et al. Minimally invasive percutaneous epicardial placement of a prototype miniature pacemaker with leadlet under direct visualization: a feasibility study in an infant porcine model. Heart Rhythm. 2019;16:1261–7. 10.1016/j.hrthm.2019.02.033. [DOI] [PubMed] [Google Scholar]


